Abstract
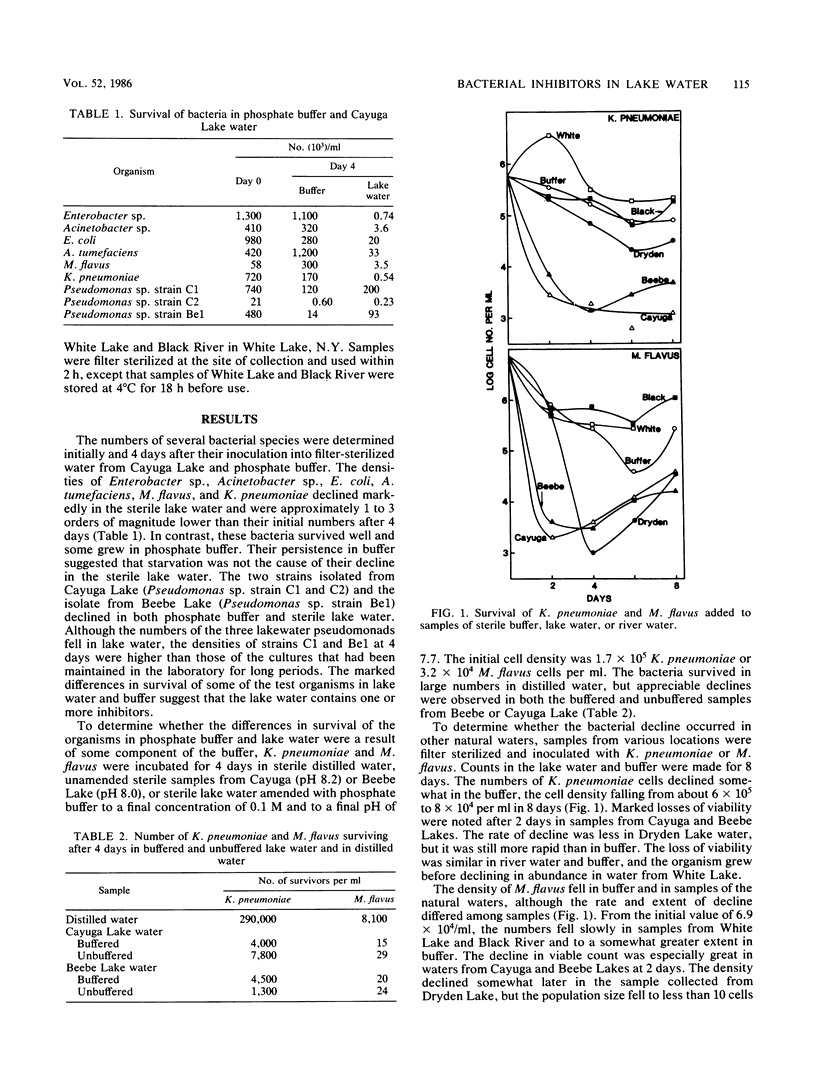
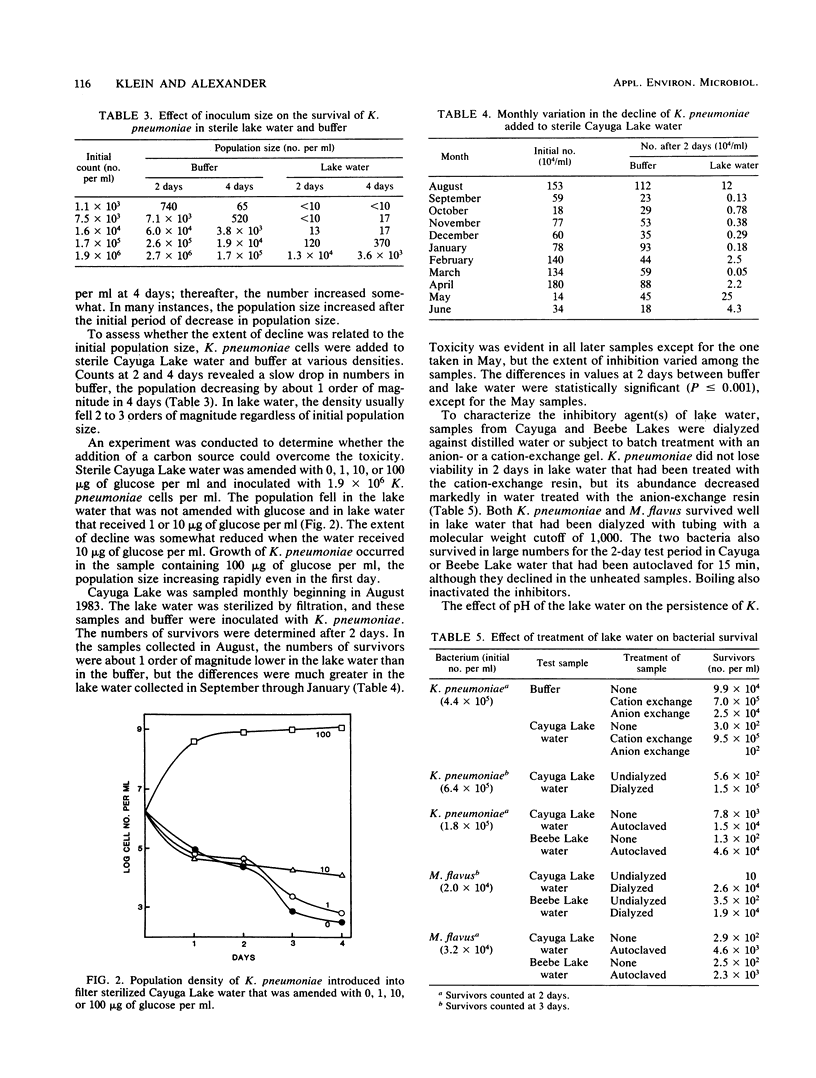
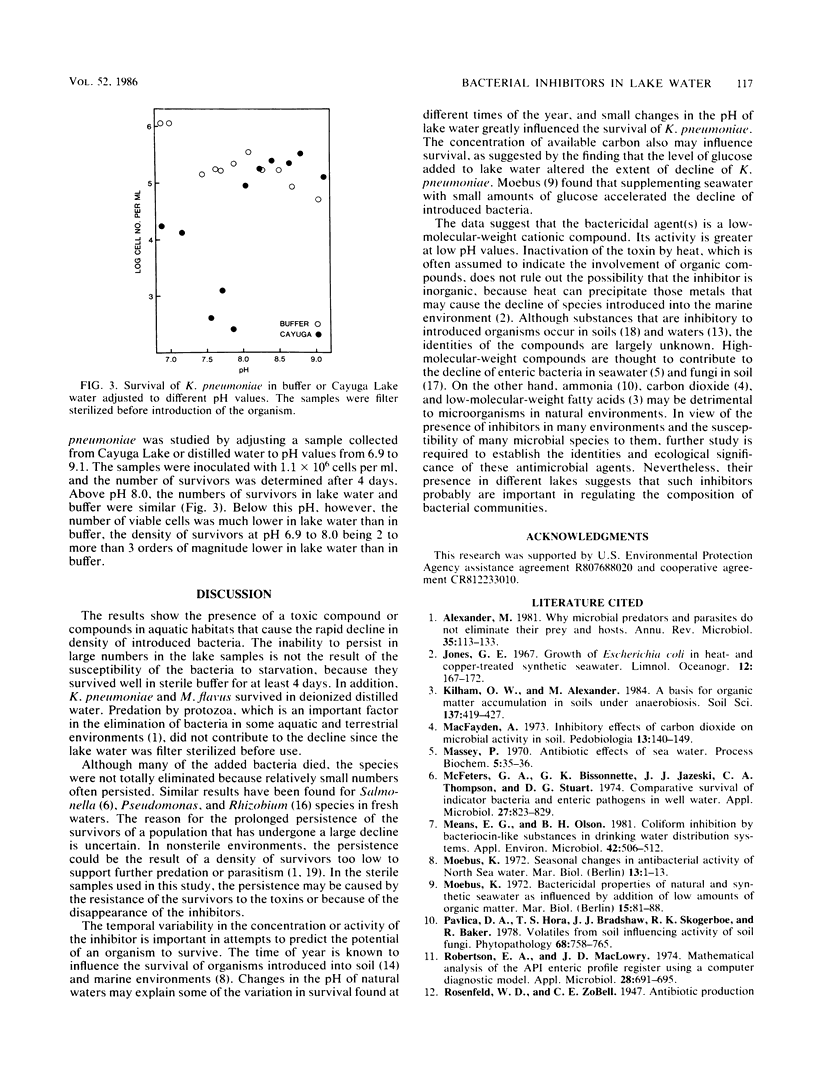
The populations of six bacterial genera fell rapidly after their addition to sterile lake water but not after their addition to buffer. The decline in numbers of two species that were studied further, Klebsiella pneumoniae and Micrococcus flavus, occurred even when the buffer was added to sterile lake water. The inhibition of K. pneumoniae by substances in lake water varied with the season of the year, and the rate and extent of decline of both species were different in sterile samples of different lakes. The extent of reduction in the density of K. pneumoniae was independent of initial population size and was diminished by the addition of 10 μg of glucose per ml of lake water. The toxin was removed from lake water by dialysis and by a cation-exchange resin but not by an anion-exchange resin, and it was destroyed by heating. The inhibition of K. pneumoniae was not evident in lake water buffered at a pH value above 8.0. We suggest that toxins may be important in determining the composition of the bacterial community of lakes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Why microbial predators and parasites do not eliminate their prey and hosts. Annu Rev Microbiol. 1981;35:113–133. doi: 10.1146/annurev.mi.35.100181.000553. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Bissonnette G. K., Jezeski J. J., Thomson C. A., Stuart D. G. Comparative survival of indicator bacteria and enteric pathogens in well water. Appl Microbiol. 1974 May;27(5):823–829. doi: 10.1128/am.27.5.823-829.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means E. G., Olson B. H. Coliform inhibition by bacteriocin-like substances in drinking water distribution systems. Appl Environ Microbiol. 1981 Sep;42(3):506–512. doi: 10.1128/aem.42.3.506-512.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. A., MacLowry J. D. Mathematical analysis of the API enteric 20 profile register using a computer diagnostic model. Appl Microbiol. 1974 Oct;28(4):691–695. doi: 10.1128/am.28.4.691-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEBURTH J. M., PRAT D. M. Anticoliform activity of sea water associated with the termination of Skeletonema costatum blooms. Trans N Y Acad Sci. 1962 Mar;24:498–501. doi: 10.1111/j.2164-0947.1962.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Sinclair J. L., Alexander M. Role of resistance to starvation in bacterial survival in sewage and lake water. Appl Environ Microbiol. 1984 Aug;48(2):410–415. doi: 10.1128/aem.48.2.410-415.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaartaja O. Inhibition of Pythium ultimum in molecular fractions from gel filtration of soil extracts. Can J Microbiol. 1974 Sep;20(9):1273–1280. doi: 10.1139/m74-196. [DOI] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


