Abstract
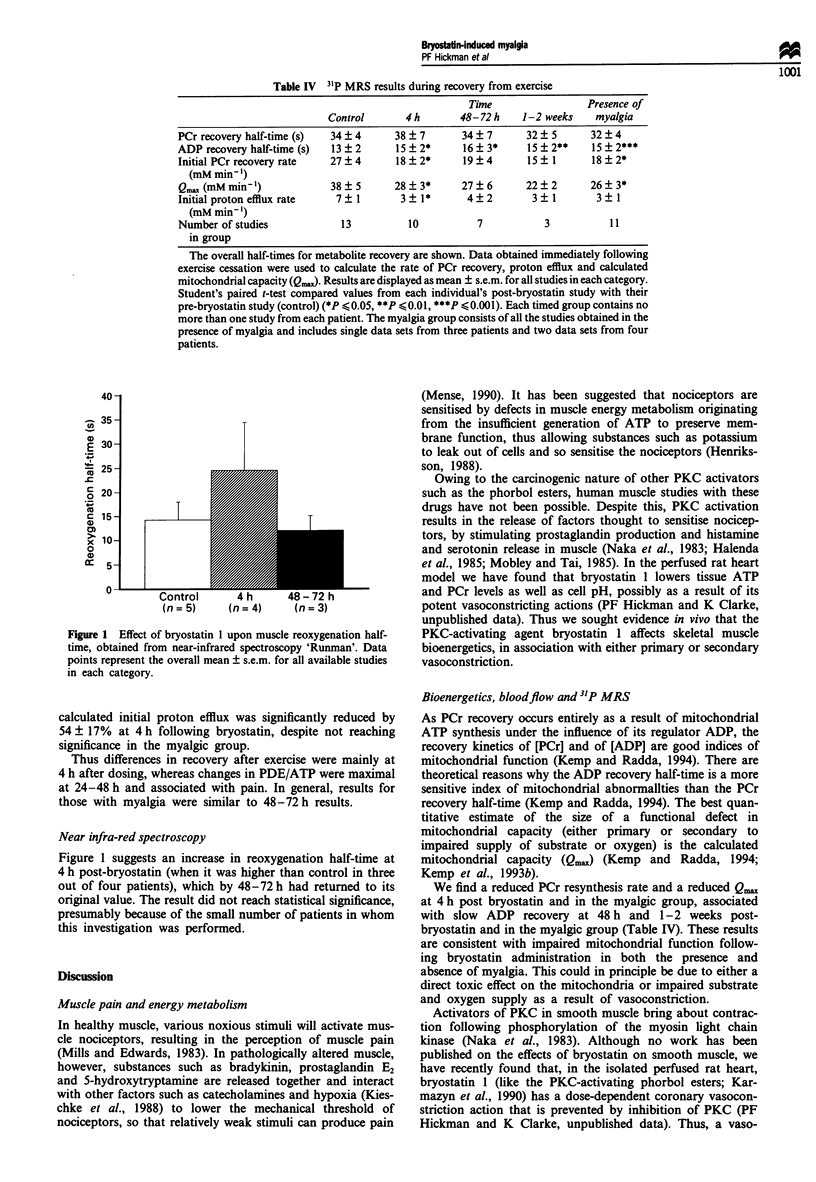
Bryostatin 1, a novel antineoplastic agent and protein kinase C (PKC) activator, has been found to induce myalgia (muscle pain) 48 h after administration in clinical trials. This is the dose-limiting toxicity and has restricted the duration of therapy in phase I trials. To investigate the mechanisms and try to increase toleration of the drug, we studied calf muscle metabolism of 14 patients at rest and during exercise and subsequent recovery using 31P magnetic resonance spectroscopy (MRS) before and 4 h, 48-72 h and 1-2 weeks following bryostatin therapy. In resting muscle there was a significant (P < 0.001) increase in the phosphodiester/adenosine 5'-triphosphate (PDE/ATP) ratio 48 h post bryostatin and in patients with myalgia compared with pre-bryostatin control studies. Following exercise, patients with myalgia showed significantly slower phosphocreatine (PCr) and ADP recovery half-time (P < or = 0.05) suggesting impaired mitochondrial (oxidative) energy production, possibly due to a direct effect on the mitochondria or secondary to reduced blood flow. The apparent proton efflux rate following exercise was significantly reduced 4 h after bryostatin (P < or = 0.05), suggesting reduced blood flow. The rate of post-exercise reoxygenation was studied in four patients by near-infrared spectroscopy 4 h post bryostatin. In three of these the rate was reduced, consistent with reduced muscle blood flow. Bryostatin 1 appeared to cause a long-lasting impairment of oxidative metabolism and proton washout from muscle, consistent with a vasoconstrictive action. Thus these studies provide evidence for two mechanisms of the dose-limiting toxicity for bryostatin. Prospective studies on the use of vasodilators to improve the tolerance of the drug should be carried out.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold D. L., Matthews P. M., Radda G. K. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984 Sep;1(3):307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- Arnold D. L., Taylor D. J., Radda G. K. Investigation of human mitochondrial myopathies by phosphorus magnetic resonance spectroscopy. Ann Neurol. 1985 Aug;18(2):189–196. doi: 10.1002/ana.410180205. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Kraft A. S. Bryostatin, a non-phorbol macrocyclic lactone, activates intact human polymorphonuclear leukocytes and binds to the phorbol ester receptor. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1109–1116. doi: 10.1016/0006-291x(85)90205-0. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eppenberger H. M. Comparison of M-line and other myofibril components during reversible phorbol ester treatment. Eur J Cell Biol. 1984 Mar;33(2):265–274. [PubMed] [Google Scholar]
- Edwards R. H., Dawson M. J., Wilkie D. R., Gordon R. E., Shaw D. Clinical use of nuclear magnetic resonance in the investigation of myopathy. Lancet. 1982 Mar 27;1(8274):725–731. doi: 10.1016/s0140-6736(82)92635-6. [DOI] [PubMed] [Google Scholar]
- Ester A., Gras J., Buera C., Planas J. Glicoforina A: estudio sobre la interconversión dimero-monomérica por efecto de la temperatura y la concentración. Sangre (Barc) 1988 Aug;33(4):265–268. [PubMed] [Google Scholar]
- Halenda S. P., Zavoico G. B., Feinstein M. B. Phorbol esters and oleoyl acetoyl glycerol enhance release of arachidonic acid in platelets stimulated by Ca2+ ionophore A23187. J Biol Chem. 1985 Oct 15;260(23):12484–12491. [PubMed] [Google Scholar]
- Hands L. J., Bore P. J., Galloway G., Morris P. J., Radda G. K. Muscle metabolism in patients with peripheral vascular disease investigated by 31P nuclear magnetic resonance spectroscopy. Clin Sci (Lond) 1986 Sep;71(3):283–290. doi: 10.1042/cs0710283. [DOI] [PubMed] [Google Scholar]
- Henriksson K. G. Muscle pain in neuromuscular disorders and primary fibromyalgia. Eur J Appl Physiol Occup Physiol. 1988;57(3):348–352. doi: 10.1007/BF00635994. [DOI] [PubMed] [Google Scholar]
- Hornung R. L., Pearson J. W., Beckwith M., Longo D. L. Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in vitro versus in vivo activity. Cancer Res. 1992 Jan 1;52(1):101–107. [PubMed] [Google Scholar]
- Karmazyn M., Watson J. E., Moffat M. P. Mechanisms for cardiac depression induced by phorbol myristate acetate in working rat hearts. Br J Pharmacol. 1990 Aug;100(4):826–830. doi: 10.1111/j.1476-5381.1990.tb14099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G. J., Radda G. K. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q. 1994 Mar;10(1):43–63. [PubMed] [Google Scholar]
- Kemp G. J., Taylor D. J., Radda G. K. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993 Jan-Feb;6(1):66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- Kemp G. J., Taylor D. J., Thompson C. H., Hands L. J., Rajagopalan B., Styles P., Radda G. K. Quantitative analysis by 31P magnetic resonance spectroscopy of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed. 1993 Sep-Oct;6(5):302–310. doi: 10.1002/nbm.1940060504. [DOI] [PubMed] [Google Scholar]
- Kemp G. J., Thompson C. H., Barnes P. R., Radda G. K. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med. 1994 Mar;31(3):248–258. doi: 10.1002/mrm.1910310303. [DOI] [PubMed] [Google Scholar]
- Kieschke J., Mense S., Prabhakar N. R. Influence of adrenaline and hypoxia on rat muscle receptors in vitro. Prog Brain Res. 1988;74:91–97. doi: 10.1016/s0079-6123(08)63003-4. [DOI] [PubMed] [Google Scholar]
- Mobley A., Tai H. H. Synergistic stimulation of thromboxane biosynthesis by calcium ionophore and phorbol ester or thrombin in human platelets. Biochem Biophys Res Commun. 1985 Jul 31;130(2):717–723. doi: 10.1016/0006-291x(85)90475-9. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A. Painful disorders of muscle. Br J Hosp Med. 1979 Oct;22(4):360, 362-5. [PubMed] [Google Scholar]
- Mori T., Yanagisawa T., Taira N. Phorbol 12,13-dibutyrate increases vascular tone but has a dual action on intracellular calcium levels in porcine coronary arteries. Naunyn Schmiedebergs Arch Pharmacol. 1990 Mar;341(3):251–255. doi: 10.1007/BF00169739. [DOI] [PubMed] [Google Scholar]
- Moses R. L., Claycomb W. C. Differentiated membrane specializations and myofibrillar breakdown and recovery in cultured adult cardiac myocytes treated with TPA and diacylglycerol. J Cell Sci. 1989 May;93(Pt 1):95–105. doi: 10.1242/jcs.93.1.95. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Philip P. A., Rea D., Thavasu P., Carmichael J., Stuart N. S., Rockett H., Talbot D. C., Ganesan T., Pettit G. R., Balkwill F. Phase I study of bryostatin 1: assessment of interleukin 6 and tumor necrosis factor alpha induction in vivo. The Cancer Research Campaign Phase I Committee. J Natl Cancer Inst. 1993 Nov 17;85(22):1812–1818. doi: 10.1093/jnci/85.22.1812. [DOI] [PubMed] [Google Scholar]
- Prendiville J., Crowther D., Thatcher N., Woll P. J., Fox B. W., McGown A., Testa N., Stern P., McDermott R., Potter M. A phase I study of intravenous bryostatin 1 in patients with advanced cancer. Br J Cancer. 1993 Aug;68(2):418–424. doi: 10.1038/bjc.1993.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Watson J. E., Karmazyn M. Concentration-dependent effects of protein kinase C-activating and -nonactivating phorbol esters on myocardial contractility, coronary resistance, energy metabolism, prostacyclin synthesis, and ultrastructure in isolated rat hearts. Effects of amiloride. Circ Res. 1991 Oct;69(4):1114–1131. doi: 10.1161/01.res.69.4.1114. [DOI] [PubMed] [Google Scholar]
- Weissman J. D., Constantinitis I., Hudgins P., Wallace D. C. 31P magnetic resonance spectroscopy suggests impaired mitochondrial function in AZT-treated HIV-infected patients. Neurology. 1992 Mar;42(3 Pt 1):619–623. doi: 10.1212/wnl.42.3.619. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Mancini D. M., McCully K., Ferraro N., Lanoce V., Chance B. Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation. 1989 Dec;80(6):1668–1674. doi: 10.1161/01.cir.80.6.1668. [DOI] [PubMed] [Google Scholar]


