Abstract
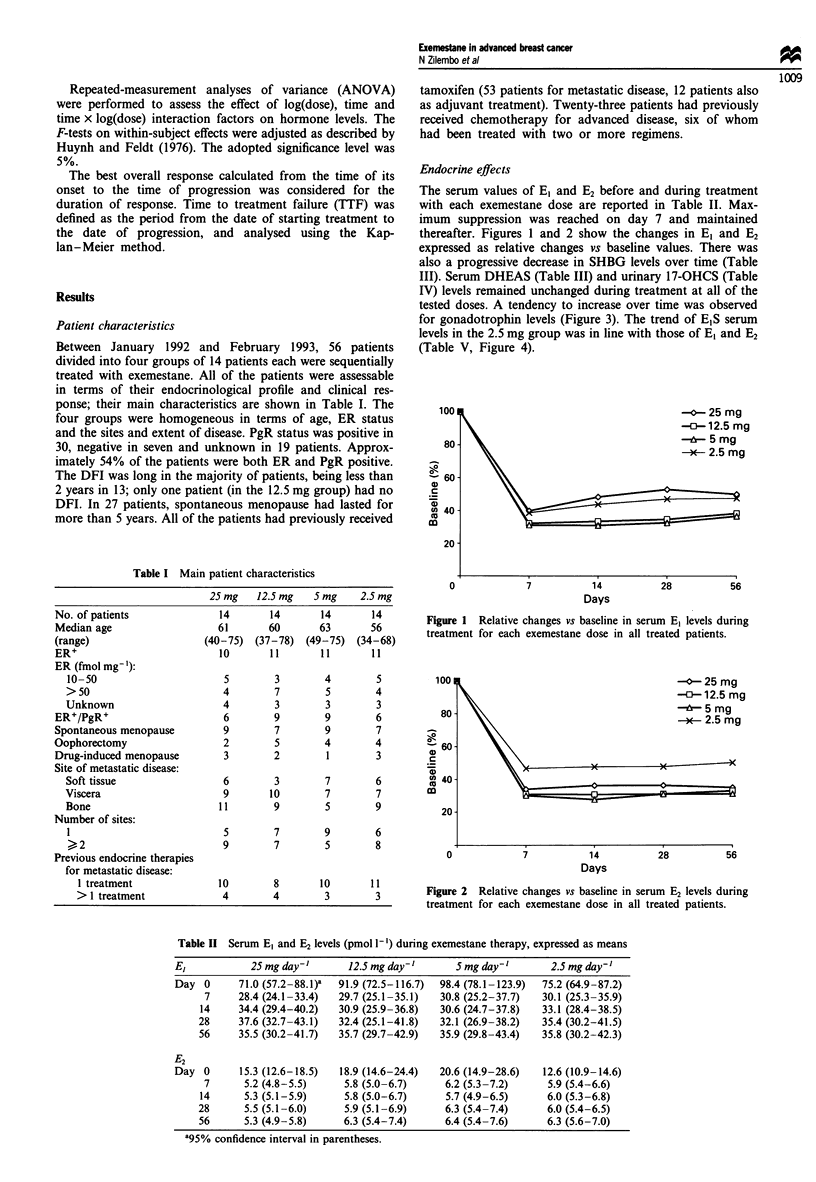
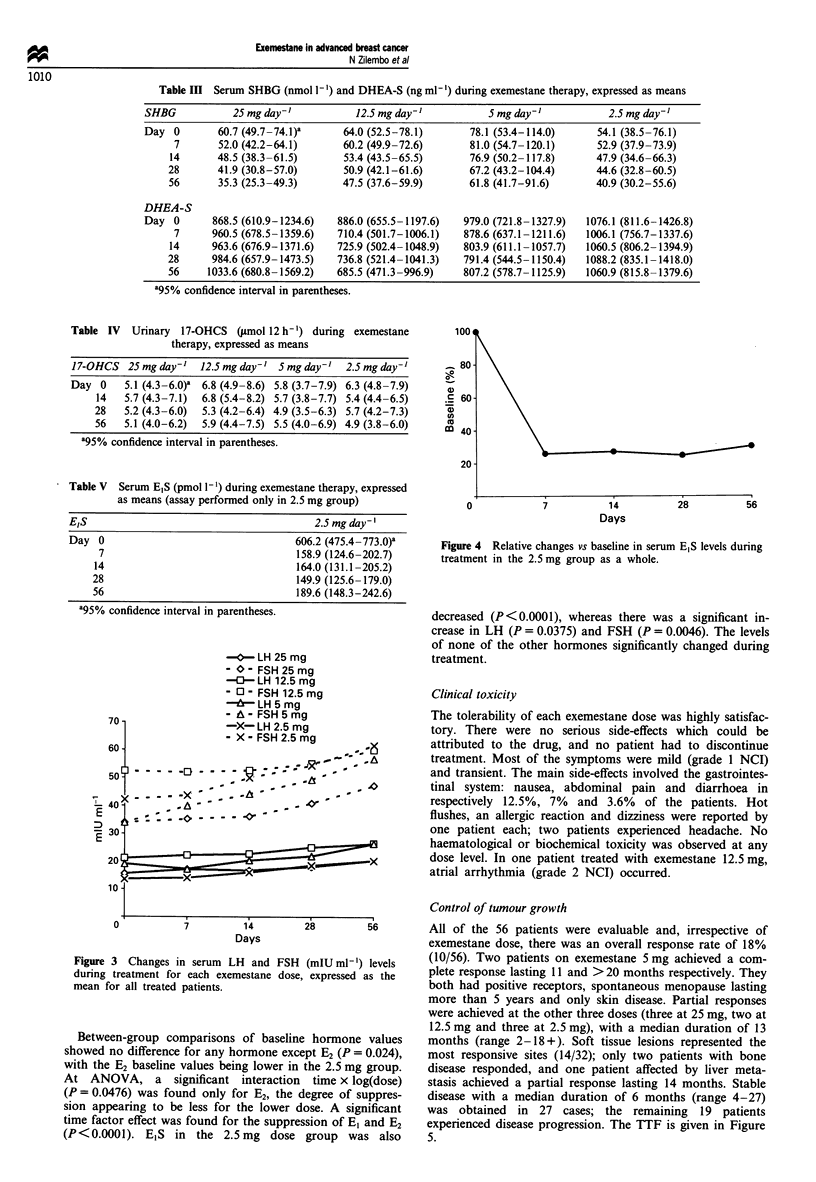
The androstenedione derivative, exemestane (FCE 24304), is a new orally active irreversible aromatase inhibitor. Fifty-six post-menopausal advanced breast cancer patients entered this study to evaluate the activity of four low exemestane doses in reducing oestrogen levels. The drug's tolerability and clinical efficacy were also assessed. Exemestane was orally administered to four consecutive groups at daily doses of 25, 12.5, 5 and 2.5 mg, and the changes in oestrogen, gonadotrophins, sex-hormone binding globulin and dehydroepiandrosterone sulphate levels were evaluated. Drug selectivity was studied by measuring 17-hydroxycorticosteroid urinary levels. After 7 days of treatment, mean oestrone and oestradiol levels had decreased by respectively 64% and 65% (a decrease which was maintained over time); in the 2.5 mg group, oestrone sulphate levels also decreased by 74%. Gonadotrophin levels were significantly higher, whereas no changes in the other serum hormone levels or any interference with adrenal synthesis were detected. Treatment tolerability was satisfactory: nausea and dyspepsia were reported in 16% of patients. The overall objective response rate was 18%. In conclusion, exemestane is effective in reducing oestrogen levels at all of the tested doses and shows interesting clinical activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajetta E., Zilembo N., Buzzoni R., Noberasco C., Celio L., Bichisao E. Efficacy and tolerability of 4-hydroxyandrostenedione (4-OHA) as first-line treatment in postmenopausal patients with breast cancer after adjuvant therapy. Cancer Treat Rev. 1993 Apr;19 (Suppl B):31–36. doi: 10.1016/0305-7372(93)90005-c. [DOI] [PubMed] [Google Scholar]
- Bajetta E., Zilembo N., Buzzoni R., Noberasco C., Di Leo A., Bartoli C., Merson M., Sacchini V., Moglia D., Celio L. Endocrinological and clinical evaluation of two doses of formestane in advanced breast cancer. Br J Cancer. 1994 Jul;70(1):145–150. doi: 10.1038/bjc.1994.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes R. C., Goss P., Dowsett M., Gazet J. C., Brodie A. 4-Hydroxyandrostenedione in treatment of postmenopausal patients with advanced breast cancer. Lancet. 1984 Dec 1;2(8414):1237–1239. doi: 10.1016/s0140-6736(84)92795-8. [DOI] [PubMed] [Google Scholar]
- Coombes R. C., Hughes S. W., Dowsett M. 4-hydroxyandrostenedione: a new treatment for postmenopausal patients with breast cancer. Eur J Cancer. 1992;28A(12):1941–1945. doi: 10.1016/0959-8049(92)90232-q. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Coombes R. C. Second generation aromatase inhibitor--4-hydroxyandrostenedione. Breast Cancer Res Treat. 1994;30(1):81–87. doi: 10.1007/BF00682742. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Cunningham D. C., Stein R. C., Evans S., Dehennin L., Hedley A., Coombes R. C. Dose-related endocrine effects and pharmacokinetics of oral and intramuscular 4-hydroxyandrostenedione in postmenopausal breast cancer patients. Cancer Res. 1989 Mar 1;49(5):1306–1312. [PubMed] [Google Scholar]
- Evans T. R., Di Salle E., Ornati G., Lassus M., Benedetti M. S., Pianezzola E., Coombes R. C. Phase I and endocrine study of exemestane (FCE 24304), a new aromatase inhibitor, in postmenopausal women. Cancer Res. 1992 Nov 1;52(21):5933–5939. [PubMed] [Google Scholar]
- Ferrari L., Zilembo N., Bajetta E., Buzzoni R., Noberasco C., Martinetti A., Celio L., Galante E., Orefice S., Cerrotta A. M. Effect of two-4-hydroxyandrostenedione doses on serum insulin-like growth factor I levels in advanced breast cancer. Breast Cancer Res Treat. 1994;30(2):127–132. doi: 10.1007/BF00666055. [DOI] [PubMed] [Google Scholar]
- Giudici D., Ornati G., Briatico G., Buzzetti F., Lombardi P., di Salle E. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): a new irreversible aromatase inhibitor. J Steroid Biochem. 1988;30(1-6):391–394. doi: 10.1016/0022-4731(88)90129-x. [DOI] [PubMed] [Google Scholar]
- Goss P. E., Powles T. J., Dowsett M., Hutchison G., Brodie A. M., Gazet J. C., Coombes R. C. Treatment of advanced postmenopausal breast cancer with an aromatase inhibitor, 4-hydroxyandrostenedione: phase II report. Cancer Res. 1986 Sep;46(9):4823–4826. [PubMed] [Google Scholar]
- Höffken K., Jonat W., Possinger K., Kölbel M., Kunz T., Wagner H., Becher R., Callies R., Friederich P., Willmanns W. Aromatase inhibition with 4-hydroxyandrostenedione in the treatment of postmenopausal patients with advanced breast cancer: a phase II study. J Clin Oncol. 1990 May;8(5):875–880. doi: 10.1200/JCO.1990.8.5.875. [DOI] [PubMed] [Google Scholar]
- Iveson T. J., Smith I. E., Ahern J., Smithers D. A., Trunet P. F., Dowsett M. Phase I study of the oral nonsteroidal aromatase inhibitor CGS 20267 in postmenopausal patients with advanced breast cancer. Cancer Res. 1993 Jan 15;53(2):266–270. [PubMed] [Google Scholar]
- Murphy D., West H. F. Urinary 17-hydroxycorticosteroids measured by gas-liquid chromatography. J Endocrinol. 1966 Dec;36(4):331–340. doi: 10.1677/joe.0.0360331. [DOI] [PubMed] [Google Scholar]
- Pickles T., Perry L., Murray P., Plowman P. 4-hydroxyandrostenedione--further clinical and extended endocrine observations. Br J Cancer. 1990 Aug;62(2):309–313. doi: 10.1038/bjc.1990.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. J., Christodoulides A., Koistinen R., Seppälä M., Teale J. D., Ghilchik M. W. The effect of endocrine therapy with medroxyprogesterone acetate, 4-hydroxyandrostenedione or tamoxifen on plasma concentrations of insulin-like growth factor (IGF)-I, IGF-II and IGFBP-1 in women with advanced breast cancer. Int J Cancer. 1992 Sep 9;52(2):208–212. doi: 10.1002/ijc.2910520209. [DOI] [PubMed] [Google Scholar]
- Stein R. C., Dowsett M., Hedley A., Davenport J., Gazet J. C., Ford H. T., Coombes R. C. Treatment of advanced breast cancer in postmenopausal women with 4-hydroxyandrostenedione. Cancer Chemother Pharmacol. 1990;26(1):75–78. doi: 10.1007/BF02940300. [DOI] [PubMed] [Google Scholar]
- Zaccheo T., Di Salle E. Effect of the irreversible aromatase inhibitor FCE 24304 on DMBA-induced mammary tumors in ovariectomized rats treated with testosterone. Cancer Chemother Pharmacol. 1989;25(2):95–98. doi: 10.1007/BF00692346. [DOI] [PubMed] [Google Scholar]
- Zaccheo T., Giudici D., Lombardi P., di Salle E. A new irreversible aromatase inhibitor, 6-methylenandrosta-1,4-diene-3,17-dione (FCE 24304): antitumor activity and endocrine effects in rats with DMBA-induced mammary tumors. Cancer Chemother Pharmacol. 1989;23(1):47–50. doi: 10.1007/BF00258457. [DOI] [PubMed] [Google Scholar]
- Zilembo N., Buzzoni R., Celio L., Noberasco C., Ferrari L., Laffranchi A., Vicario G., Dolci S., Bajetta E. Formestane as treatment of advanced breast cancer in elderly women. Tumori. 1994 Dec 31;80(6):433–437. doi: 10.1177/030089169408000605. [DOI] [PubMed] [Google Scholar]
- di Salle E., Giudici D., Briatico G., Ornati G. Novel irreversible aromatase inhibitors. Ann N Y Acad Sci. 1990;595:357–367. doi: 10.1111/j.1749-6632.1990.tb34309.x. [DOI] [PubMed] [Google Scholar]
- di Salle E., Ornati G., Giudici D., Lassus M., Evans T. R., Coombes R. C. Exemestane (FCE 24304), a new steroidal aromatase inhibitor. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):137–143. doi: 10.1016/0960-0760(92)90198-r. [DOI] [PubMed] [Google Scholar]


