Abstract
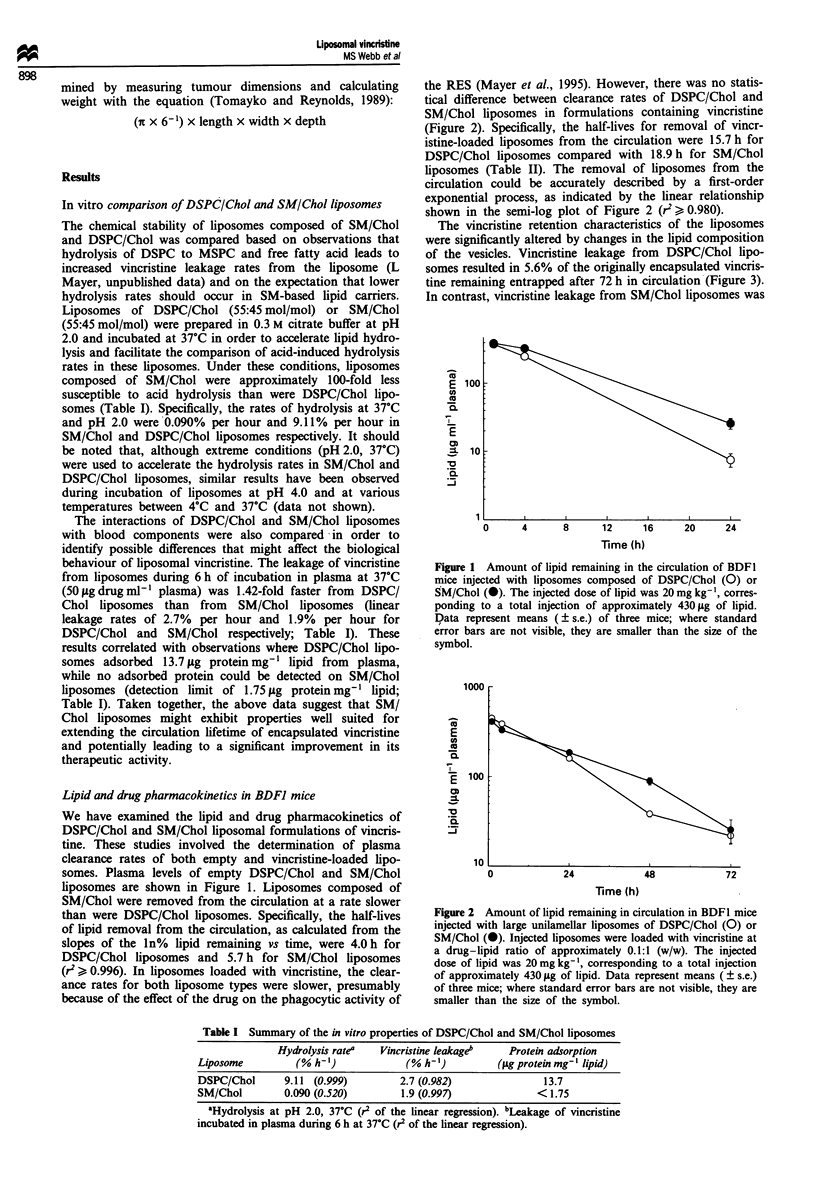
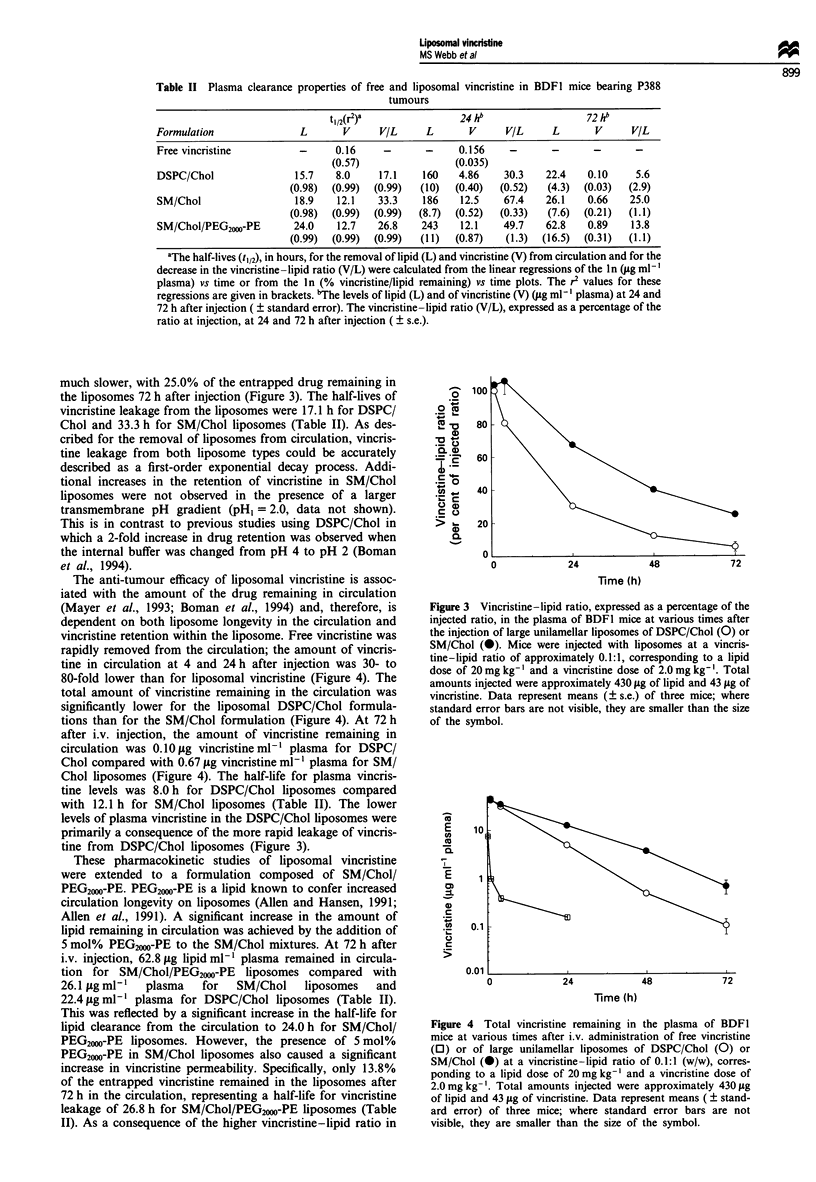
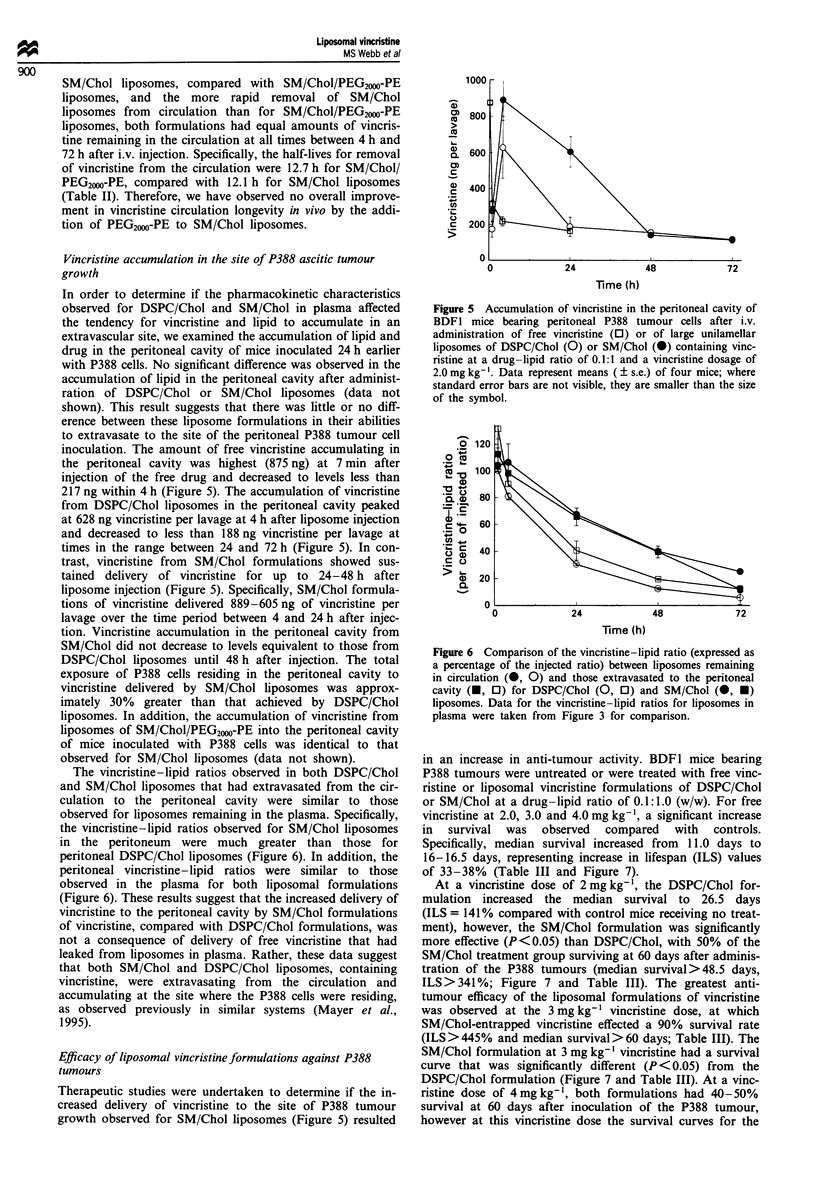
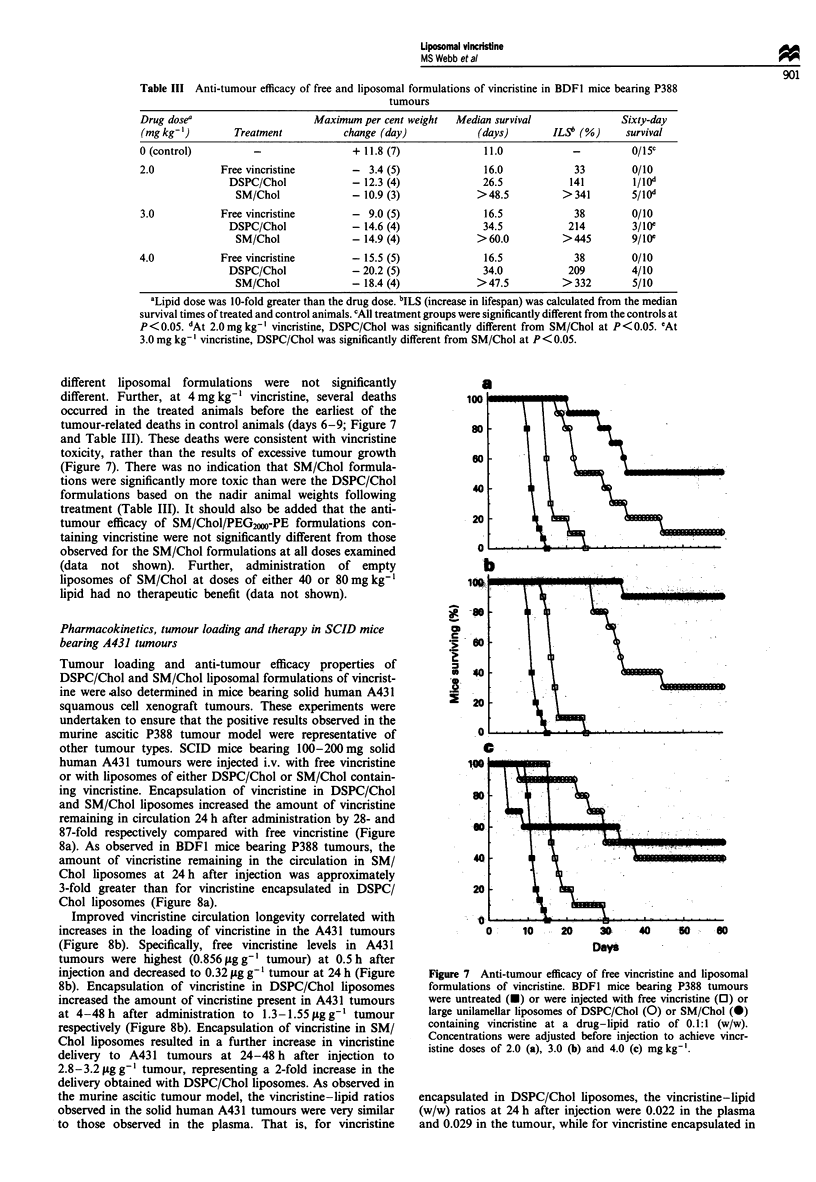
This study reports on the development of a liposomal formulation of vincristine with significantly enhanced stability and biological properties. The in vitro and in vivo pharmacokinetic, tumour delivery and efficacy properties of liposomal vincristine formulations based on sphingomyelin (SM) and cholesterol were compared with liposomes composed of distearoylphosphatidylcholine (DSPC) and cholesterol. SM/cholesterol liposomes had significantly greater in vitro stability than did similar DSPC/cholesterol liposomes. SM/cholesterol liposomes also had significantly improved biological properties compared with DSPC/cholesterol. Specifically, SM/cholesterol liposomes administered intravenously retained 25% of the entrapped vincristine after 72 h in the circulation, compared with 5% retention in DSPC/cholesterol liposomes. The improved retention properties of SM/cholesterol liposomes resulted in plasma vincristine levels 7-fold higher than in DSPC/cholesterol liposomes. The improved circulation lifetime of vincristine in SM/cholesterol liposomes correlated with increased vincristine accumulation in peritoneal ascitic murine P388 tumours and in subcutaneous solid A431 human xenograft tumours. Increased vincristine delivery to tumours was also accompanied by increased anti-tumour efficacy. Treatment with SM/cholesterol liposomal formulations of vincristine resulted in greater than 50% cures in mice bearing ascitic P388 tumours, an activity that could not be achieved with the DSPC/cholesterol formulation. Similarly, treatment of mice with severe combined immunodeficiency (SCID) bearing solid human A431 xenograft tumours with SM/cholesterol vincristine formulations delayed the time required for 100% increase in tumour mass to > 40 days, compared with 5 days, 7 days and 14 days for mice receiving no treatment or treatment with free vincristine or DSPC/cholesterol formulations of vincristine respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987 Oct 19;223(1):42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Hansen C., Martin F., Redemann C., Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991 Jul 1;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991 Sep 30;1068(2):133–141. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Hansen C., Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989 May 19;981(1):27–35. doi: 10.1016/0005-2736(89)90078-3. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Murray L., MacKeigan S., Shah M. Chronic liposome administration in mice: effects on reticuloendothelial function and tissue distribution. J Pharmacol Exp Ther. 1984 Apr;229(1):267–275. [PubMed] [Google Scholar]
- Allen T. M., Smuckler E. A. Liver pathology accompanying chronic liposome administration in mouse. Res Commun Chem Pathol Pharmacol. 1985 Nov;50(2):281–290. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bally M. B., Masin D., Nayar R., Cullis P. R., Mayer L. D. Transfer of liposomal drug carriers from the blood to the peritoneal cavity of normal and ascitic tumor-bearing mice. Cancer Chemother Pharmacol. 1994;34(2):137–146. doi: 10.1007/BF00685931. [DOI] [PubMed] [Google Scholar]
- Bally M. B., Nayar R., Masin D., Hope M. J., Cullis P. R., Mayer L. D. Liposomes with entrapped doxorubicin exhibit extended blood residence times. Biochim Biophys Acta. 1990 Mar 30;1023(1):133–139. doi: 10.1016/0005-2736(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Blume G., Cevc G. Molecular mechanism of the lipid vesicle longevity in vivo. Biochim Biophys Acta. 1993 Mar 14;1146(2):157–168. doi: 10.1016/0005-2736(93)90351-y. [DOI] [PubMed] [Google Scholar]
- Boman N. L., Masin D., Mayer L. D., Cullis P. R., Bally M. B. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994 Jun 1;54(11):2830–2833. [PubMed] [Google Scholar]
- Chonn A., Semple S. C., Cullis P. R. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J Biol Chem. 1992 Sep 15;267(26):18759–18765. [PubMed] [Google Scholar]
- Gabizon A. A. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Res. 1992 Feb 15;52(4):891–896. [PubMed] [Google Scholar]
- Gabizon A., Catane R., Uziely B., Kaufman B., Safra T., Cohen R., Martin F., Huang A., Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994 Feb 15;54(4):987–992. [PubMed] [Google Scholar]
- Horton J. K., Houghton P. J., Houghton J. A. Relationships between tumor responsiveness, vincristine pharmacokinetics and arrest of mitosis in human tumor xenografts. Biochem Pharmacol. 1988 Oct 15;37(20):3995–4000. doi: 10.1016/0006-2952(88)90085-8. [DOI] [PubMed] [Google Scholar]
- Kanter P. M., Klaich G. M., Bullard G. A., King J. M., Bally M. B., Mayer L. D. Liposome encapsulated vincristine: preclinical toxicologic and pharmacologic comparison with free vincristine and empty liposomes in mice, rats and dogs. Anticancer Drugs. 1994 Oct;5(5):579–590. doi: 10.1097/00001813-199410000-00010. [DOI] [PubMed] [Google Scholar]
- Layton D., Trouet A. A comparison of the therapeutic effects of free and liposomally encapsulated vincristine in leukemic mice. Eur J Cancer. 1980 Jul;16(7):945–950. doi: 10.1016/0014-2964(80)90333-3. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Bally M. B., Loughrey H., Masin D., Cullis P. R. Liposomal vincristine preparations which exhibit decreased drug toxicity and increased activity against murine L1210 and P388 tumors. Cancer Res. 1990 Feb 1;50(3):575–579. [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985 Jul 11;817(1):193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Masin D., Nayar R., Boman N. L., Bally M. B. Pharmacology of liposomal vincristine in mice bearing L1210 ascitic and B16/BL6 solid tumours. Br J Cancer. 1995 Mar;71(3):482–488. doi: 10.1038/bjc.1995.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. D., Nayar R., Thies R. L., Boman N. L., Cullis P. R., Bally M. B. Identification of vesicle properties that enhance the antitumour activity of liposomal vincristine against murine L1210 leukemia. Cancer Chemother Pharmacol. 1993;33(1):17–24. doi: 10.1007/BF00686017. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Tai L. C., Bally M. B., Mitilenes G. N., Ginsberg R. S., Cullis P. R. Characterization of liposomal systems containing doxorubicin entrapped in response to pH gradients. Biochim Biophys Acta. 1990 Jun 27;1025(2):143–151. doi: 10.1016/0005-2736(90)90091-2. [DOI] [PubMed] [Google Scholar]
- Parr M. J., Bally M. B., Cullis P. R. The presence of GM1 in liposomes with entrapped doxorubicin does not prevent RES blockade. Biochim Biophys Acta. 1993 Jun 12;1168(2):249–252. doi: 10.1016/0005-2760(93)90132-s. [DOI] [PubMed] [Google Scholar]
- Tomayko M. M., Reynolds C. P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- Vaage J., Donovan D., Mayhew E., Uster P., Woodle M. Therapy of mouse mammary carcinomas with vincristine and doxorubicin encapsulated in sterically stabilized liposomes. Int J Cancer. 1993 Jul 30;54(6):959–964. doi: 10.1002/ijc.2910540616. [DOI] [PubMed] [Google Scholar]
- Weereratne E. A., Gregoriadis G., Crow J. Toxicity of sphingomyelin-containing liposomes after chronic injection into mice. Br J Exp Pathol. 1983 Dec;64(6):670–676. [PMC free article] [PubMed] [Google Scholar]
- Yuan F., Leunig M., Huang S. K., Berk D. A., Papahadjopoulos D., Jain R. K. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994 Jul 1;54(13):3352–3356. [PubMed] [Google Scholar]


