Abstract
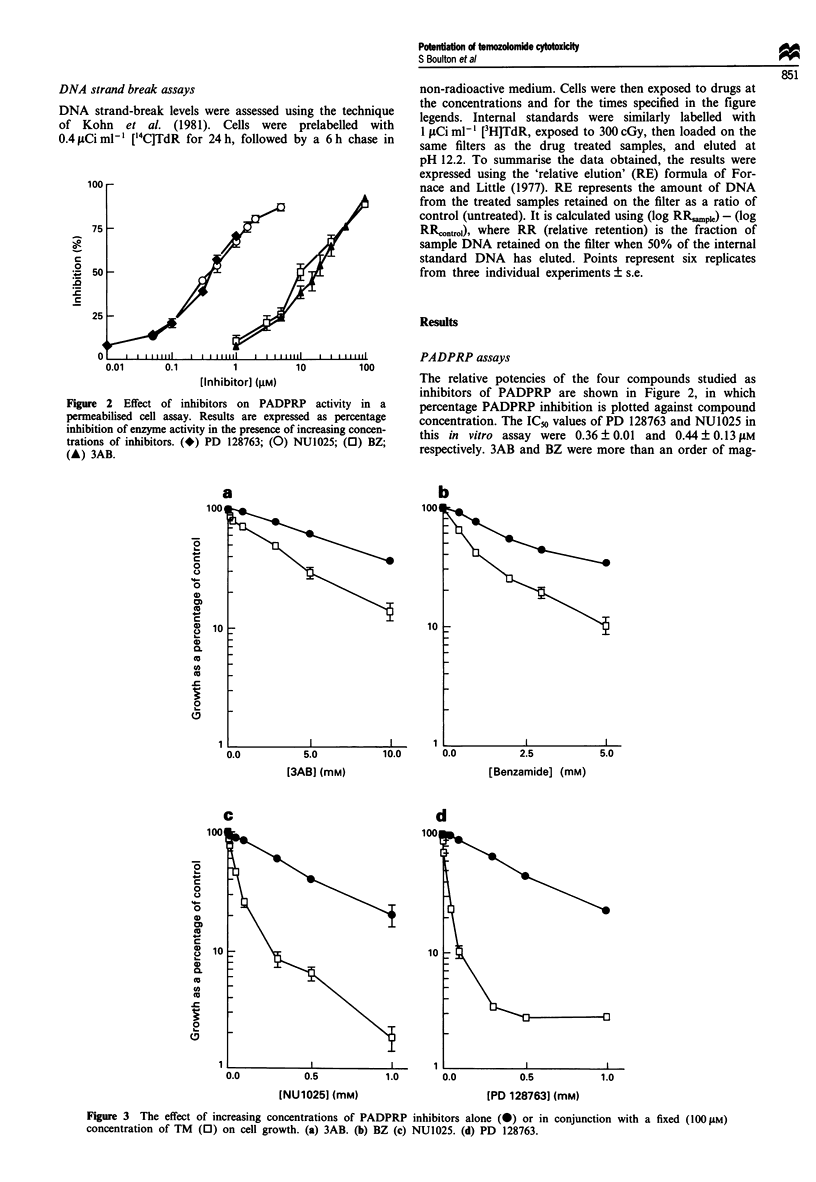
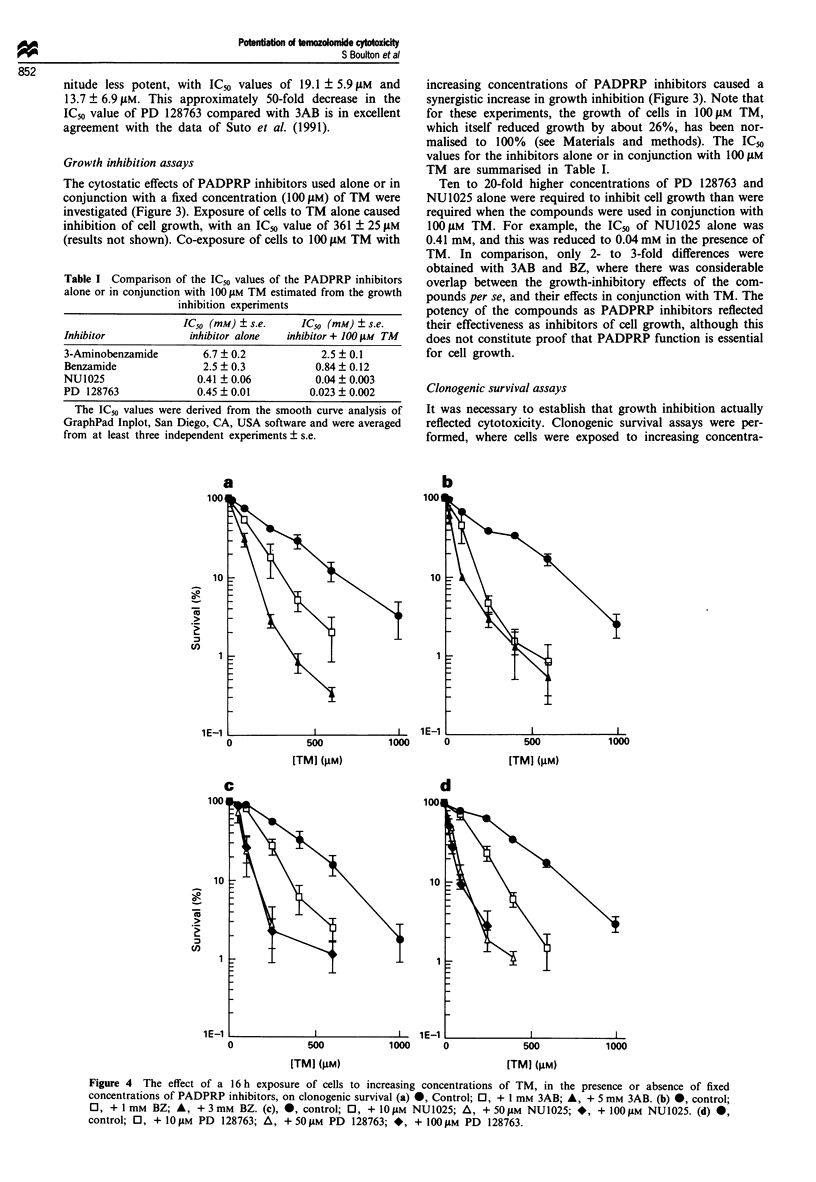
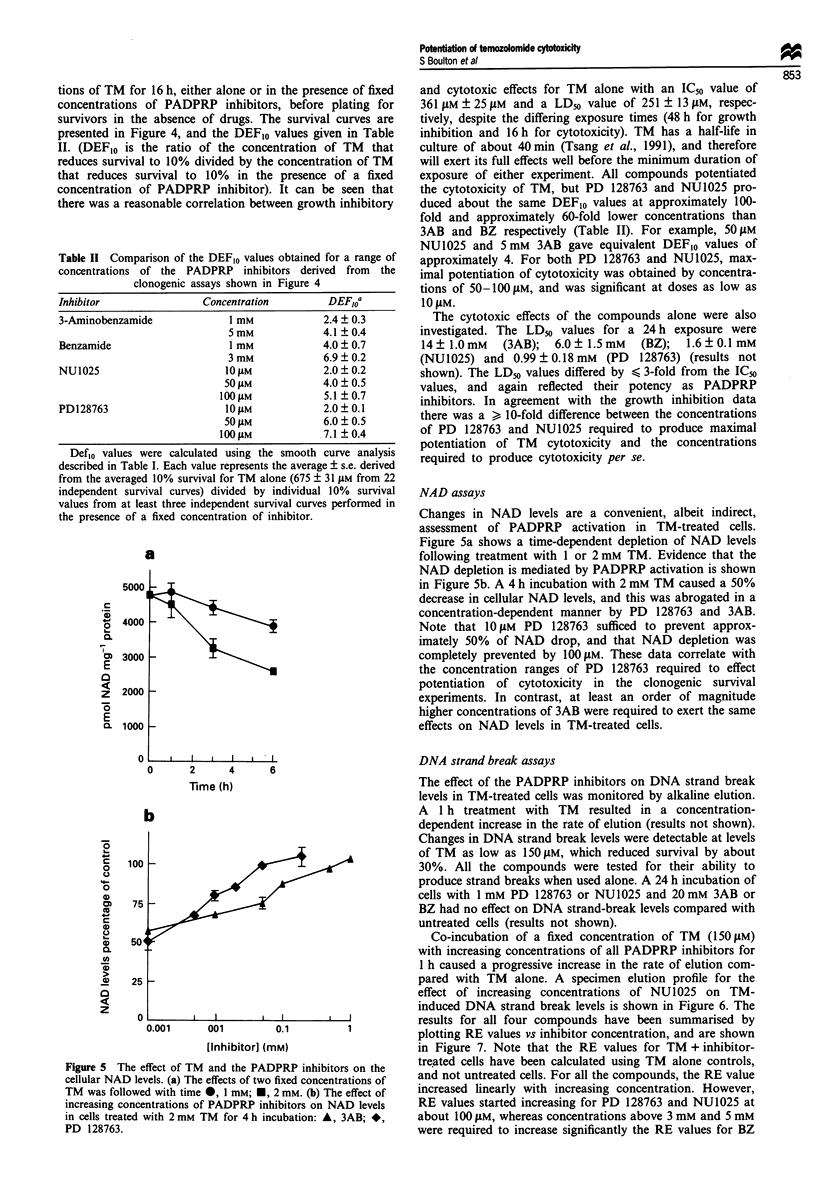
Four poly(ADP-ribose) polymerase (PADPRP) inhibitors [3-aminobenzamide, benzamide, 3,4-dihydro-5-methoxyisoquinolin-1(2H)-one (PD 128763) and 8-hydroxy-2-methylquinazolin-4(3H)-one (NU1025)] were compared with respect to their effects on a number of biological end points. The following parameters were assessed: their ability to inhibit the enzyme in permeabilised L1210 cells; their ability to potentiate the cytotoxicity of temozolomide (including the cytotoxicity of the compounds per se); their ability to increase net levels of temozolomide-induced DNA strand breaks and inhibit temozolomide-induced NAD depletion. PD 128763 and NU1025 were equipotent as PADPRP inhibitors, and 40- and 50-fold more potent than benzamide and 3-aminobenzamide respectively. All the compounds acted in a concentration-dependent manner to potentiate the cytotoxicity and increase DNA strand break levels in cells treated with temozolomide. There was an excellent correlation between the potency of the compounds as PADPRP inhibitors and their effects on cell survival and DNA repair. Temozolomide treatment caused a decrease in cellular NAD levels, and this was abolished by the PADPRP inhibitors. In conclusion, the new generation of PADPRP inhibitors are at least 50-fold more effective than 3-aminobenzamide as chemopotentiators, and can be used at micromolar rather than millimolar concentrations in intact cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Höfferer L., Kleczkowska H. E., Malanga M., Naegeli H., Panzeter P., Realini C. Histone shuttle driven by the automodification cycle of poly(ADP-ribose)polymerase. Environ Mol Mutagen. 1993;22(4):278–282. doi: 10.1002/em.2850220417. [DOI] [PubMed] [Google Scholar]
- Banasik M., Komura H., Shimoyama M., Ueda K. Specific inhibitors of poly(ADP-ribose) synthetase and mono(ADP-ribosyl)transferase. J Biol Chem. 1992 Jan 25;267(3):1569–1575. [PubMed] [Google Scholar]
- Boulikas T. Relation between carcinogenesis, chromatin structure and poly(ADP-ribosylation) (review). Anticancer Res. 1991 Mar-Apr;11(2):489–527. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Cheng M. F., Berger S. J., Berger N. A. Alkylating agent hypersensitivity in poly(adenosine diphosphate-ribose) polymerase deficient cell lines. Cancer Commun. 1991 Mar;3(3):71–75. doi: 10.3727/095535491820873551. [DOI] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Denny B. J., Wheelhouse R. T., Stevens M. F., Tsang L. L., Slack J. A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994 Aug 9;33(31):9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Ferro A. M., Higgins N. P., Olivera B. M. Poly(ADP-ribosylation) of a DNA topoisomerase. J Biol Chem. 1983 May 25;258(10):6000–6003. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Little J. B. DNA crosslinking induced by x-rays and chemical agents. Biochim Biophys Acta. 1977 Aug 16;477(4):343–355. doi: 10.1016/0005-2787(77)90253-2. [DOI] [PubMed] [Google Scholar]
- Grube K., Küpper J. H., Bürkle A. Direct stimulation of poly(ADP ribose) polymerase in permeabilized cells by double-stranded DNA oligomers. Anal Biochem. 1991 Mar 2;193(2):236–239. doi: 10.1016/0003-2697(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Halldorsson H., Gray D. A., Shall S. Poly (ADP-ribose) polymerase activity in nucleotide permeable cells. FEBS Lett. 1978 Jan 15;85(2):349–352. doi: 10.1016/0014-5793(78)80489-x. [DOI] [PubMed] [Google Scholar]
- Hunting D. J., Gowans B. J., Henderson J. F. Specificity of inhibitors of poly(ADP-ribose) synthesis. Effects on nucleotide metabolism in cultured cells. Mol Pharmacol. 1985 Aug;28(2):200–206. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- MacLaren R. A., Witmer M. V., Richardson E., Stamato T. D. Isolation of Chinese hamster ovary cells with reduced poly(ADP-ribose) polymerase activity. Mutat Res. 1990 Aug;231(2):265–274. doi: 10.1016/0027-5107(90)90032-y. [DOI] [PubMed] [Google Scholar]
- Molinete M., Vermeulen W., Bürkle A., Ménissier-de Murcia J., Küpper J. H., Hoeijmakers J. H., de Murcia G. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. EMBO J. 1993 May;12(5):2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K., Willmore E., Harris A. L., Durkacz B. W. Correlation of enhanced 6-mercaptopurine cytotoxicity with increased phosphoribosylpyrophosphate levels in Chinese hamster ovary cells treated with 3-aminobenzamide. Cancer Res. 1990 Apr 1;50(7):1992–1996. [PubMed] [Google Scholar]
- Newlands E. S., Blackledge G. R., Slack J. A., Rustin G. J., Smith D. B., Stuart N. S., Quarterman C. P., Hoffman R., Stevens M. F., Brampton M. H. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer. 1992 Feb;65(2):287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisselbaum J. S., Green S. A simple ultramicro method for determination of pyridine nucleotides in tissues. Anal Biochem. 1969 Feb;27(2):212–217. doi: 10.1016/0003-2697(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin P. W., Jacobson E. L., Benjamin R. C., Moss J., Jacobson M. K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem. 1989 Mar 15;264(8):4312–4317. [PubMed] [Google Scholar]
- Satoh M. S., Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992 Mar 26;356(6367):356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Poirier G. G., Lindahl T. NAD(+)-dependent repair of damaged DNA by human cell extracts. J Biol Chem. 1993 Mar 15;268(8):5480–5487. [PubMed] [Google Scholar]
- Sebolt-Leopold J. S., Scavone S. V. Enhancement of alkylating agent activity in vitro by PD 128763, a potent poly(ADP-ribose) synthetase inhibitor. Int J Radiat Oncol Biol Phys. 1992;22(3):619–621. doi: 10.1016/0360-3016(92)90889-p. [DOI] [PubMed] [Google Scholar]
- Smulson M., Istock N., Ding R., Cherney B. Deletion mutants of poly(ADP-ribose) polymerase support a model of cyclic association and dissociation of enzyme from DNA ends during DNA repair. Biochemistry. 1994 May 24;33(20):6186–6191. doi: 10.1021/bi00186a018. [DOI] [PubMed] [Google Scholar]
- Stevens M. F., Hickman J. A., Langdon S. P., Chubb D., Vickers L., Stone R., Baig G., Goddard C., Gibson N. W., Slack J. A. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987 Nov 15;47(22):5846–5852. [PubMed] [Google Scholar]
- Suto M. J., Turner W. R., Arundel-Suto C. M., Werbel L. M., Sebolt-Leopold J. S. Dihydroisoquinolinones: the design and synthesis of a new series of potent inhibitors of poly(ADP-ribose) polymerase. Anticancer Drug Des. 1991 May;6(2):107–117. [PubMed] [Google Scholar]
- Tsang L. L., Quarterman C. P., Gescher A., Slack J. A. Comparison of the cytotoxicity in vitro of temozolomide and dacarbazine, prodrugs of 3-methyl-(triazen-1-yl)imidazole-4-carboxamide. Cancer Chemother Pharmacol. 1991;27(5):342–346. doi: 10.1007/BF00688855. [DOI] [PubMed] [Google Scholar]
- Witmer M. V., Aboul-Ela N., Jacobson M. K., Stamato T. D. Increased sensitivity to DNA-alkylating agents in CHO mutants with decreased poly(ADP-ribose) polymerase activity. Mutat Res. 1994 May;314(3):249–260. doi: 10.1016/0921-8777(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Yoshihara K., Itaya A., Tanaka Y., Ohashi Y., Ito K., Teraoka H., Tsukada K., Matsukage A., Kamiya T. Inhibition of DNA polymerase alpha, DNA polymerase beta, terminal deoxynucleotidyl transferase, and DNA ligase II by poly(ADP-ribosyl)ation reaction in vitro. Biochem Biophys Res Commun. 1985 Apr 16;128(1):61–67. doi: 10.1016/0006-291x(85)91644-4. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Ménissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994 Apr;19(4):172–176. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]


