Abstract
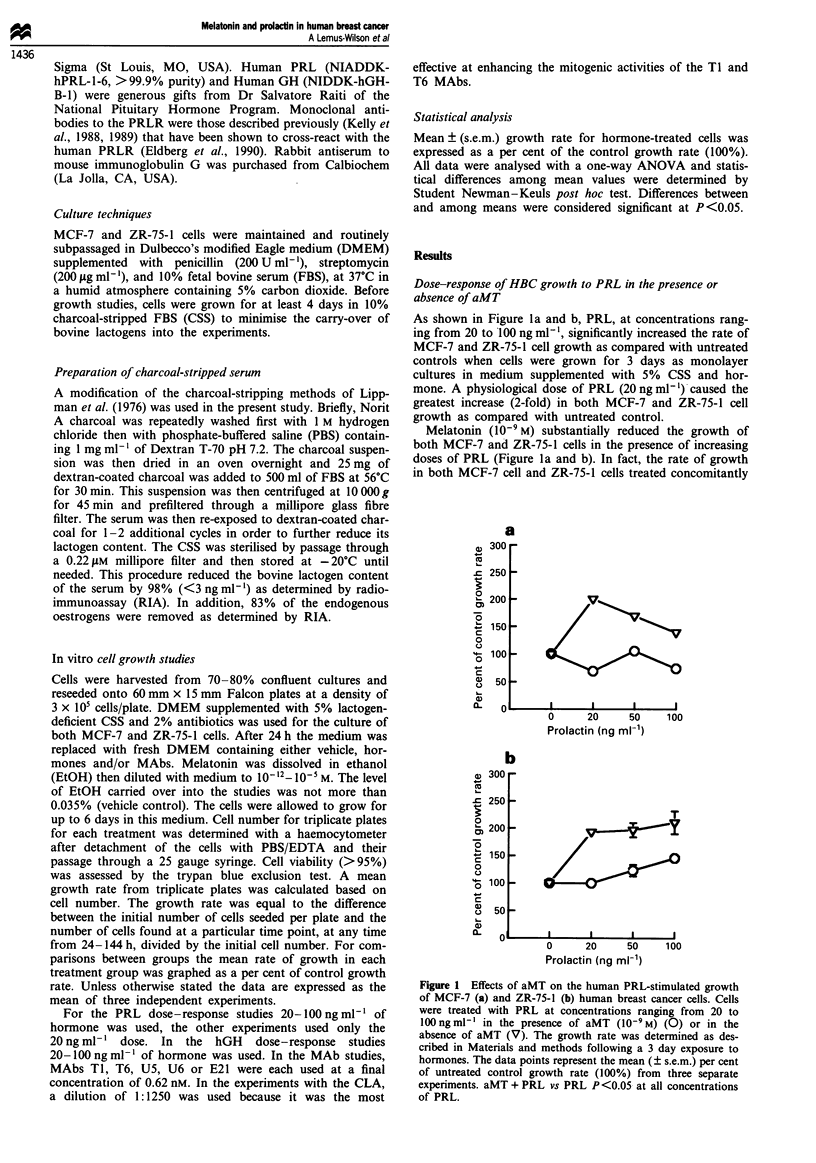
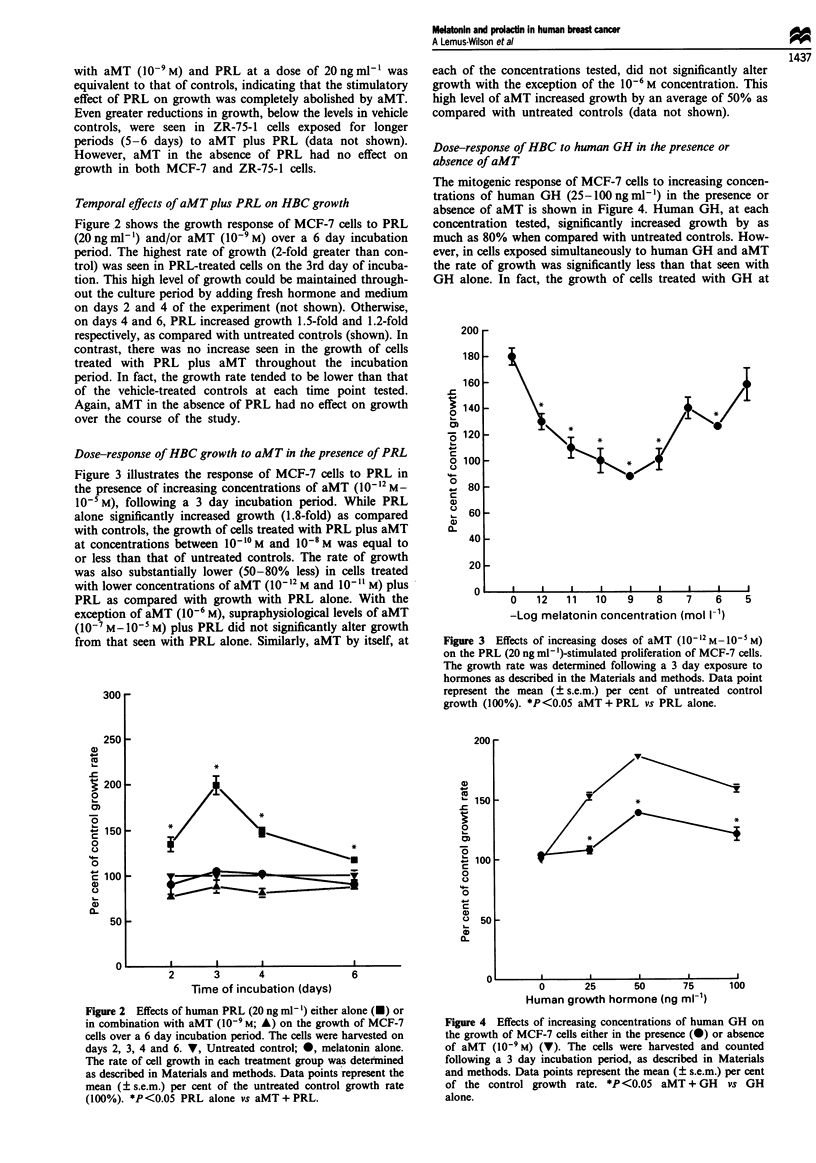
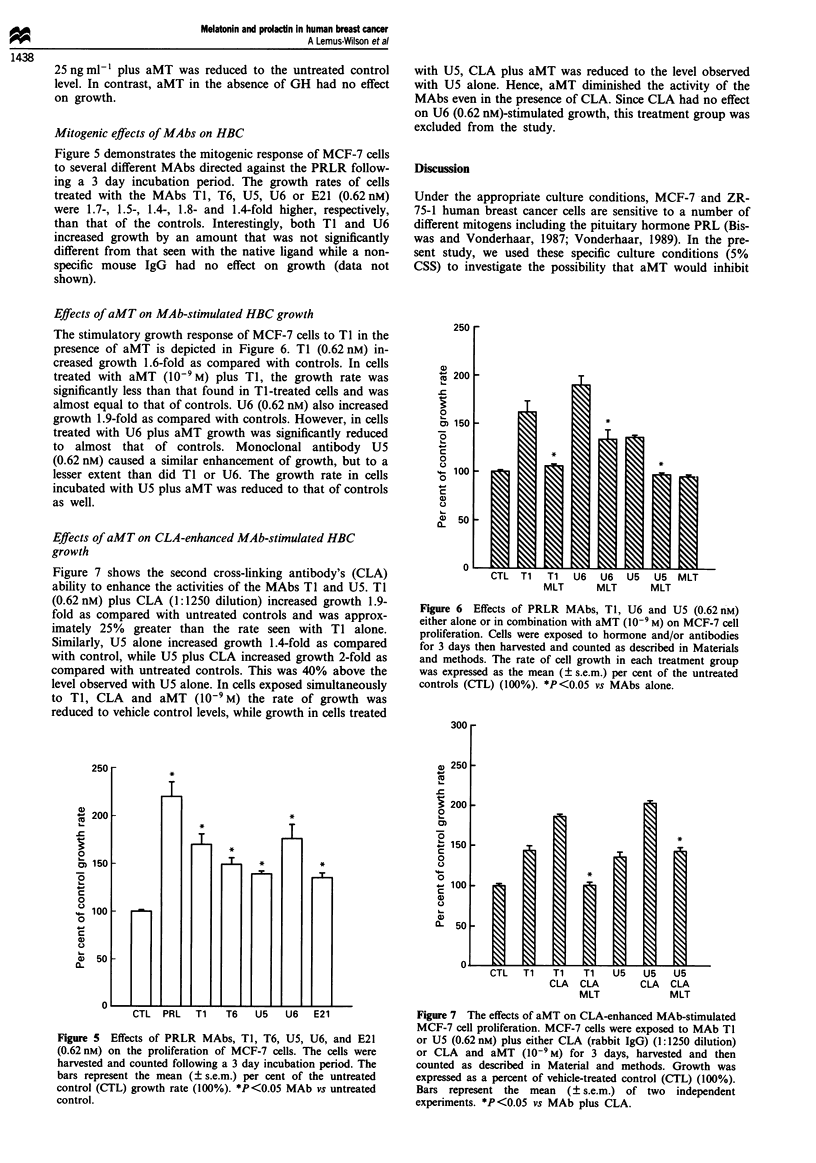
Melatonin (aMT) appears to be a potentially important oncostatic substance that can block the mitogenic effects of tumour-promoting hormones and growth factors such as oestradiol and epidermal growth factor, in vitro. In the present study, we examined the possibility that aMT would also inhibit the stimulatory effects of the tumour-promoter prolactin (PRL) on MCF-7 and ZR75-1 human breast cancer cell (HBC) growth under 5% charcoal-stripped fetal bovine serum culture conditions. Human PRL (10-100 ng ml-1) stimulated the rate of MCF-7 and ZR-75-1 HBC growth up to 2-fold above that of untreated controls. Melatonin, at concentrations between 10(-12) M and 10(-5)M, diminished and at physiological levels completely abolished PRL's mitogenic activity, but had no effect on growth in the absence of PRL. The mitogenic effects of human growth hormone (hGH), a PRL-related hormone, and also of several monoclonal antibodies (MAbs) against the PRL receptor (PRLR), were also abrogated by physiological concentrations of aMT. Additionally, aMT blocked the enhancement of MAb mitogenic activity induced by a second 'cross-linking' antibody (CLA). These findings indicate that aMT interrupts the PRLR-mediated growth signal in HBC and suggest that the oncostatic activity of aMT may also be linked with an antagonism of PRL's actions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert C., Janiaud P., Lecalvez J. Effect of pinealectomy and melatonin on mammary tumor growth in Sprague-Dawley rats under different conditions of lighting. J Neural Transm. 1980;47(2):121–130. doi: 10.1007/BF01670163. [DOI] [PubMed] [Google Scholar]
- Biswas R., Vonderhaar B. K. Antiestrogen inhibition of prolactin-induced growth of the Nb2 rat lymphoma cell line. Cancer Res. 1989 Nov 15;49(22):6295–6299. [PubMed] [Google Scholar]
- Blask D. E., Hill S. M. Effects of melatonin on cancer: studies on MCF-7 human breast cancer cells in culture. J Neural Transm Suppl. 1986;21:433–449. [PubMed] [Google Scholar]
- Blask D. E., Lemus-Wilson A. M., Wilson S. T. Breast cancer: a model system for studying the neuroendocrine role of pineal melatonin in oncology. Biochem Soc Trans. 1992 May;20(2):309–311. doi: 10.1042/bst0200309. [DOI] [PubMed] [Google Scholar]
- Blask D. E., Pelletier D. B., Hill S. M., Lemus-Wilson A., Grosso D. S., Wilson S. T., Wise M. E. Pineal melatonin inhibition of tumor promotion in the N-nitroso-N-methylurea model of mammary carcinogenesis: potential involvement of antiestrogenic mechanisms in vivo. J Cancer Res Clin Oncol. 1991;117(6):526–532. doi: 10.1007/BF01613283. [DOI] [PubMed] [Google Scholar]
- Bonneterre J., Peyrat J. P., Beuscart R., Lefebvre J., Demaille A. Prognostic significance of prolactin receptors in human breast cancer. Cancer Res. 1987 Sep 1;47(17):4724–4728. [PubMed] [Google Scholar]
- Boutin J. M., Edery M., Shirota M., Jolicoeur C., Lesueur L., Ali S., Gould D., Djiane J., Kelly P. A. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989 Sep;3(9):1455–1461. doi: 10.1210/mend-3-9-1455. [DOI] [PubMed] [Google Scholar]
- Cos S., Blask D. E. Effects of the pineal hormone melatonin on the anchorage-independent growth of human breast cancer cells (MCF-7) in a clonogenic culture system. Cancer Lett. 1990 Apr 20;50(2):115–119. doi: 10.1016/0304-3835(90)90240-x. [DOI] [PubMed] [Google Scholar]
- Cos S., Blask D. E. Melatonin modulates growth factor activity in MCF-7 human breast cancer cells. J Pineal Res. 1994 Aug;17(1):25–32. doi: 10.1111/j.1600-079x.1994.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Elberg G., Kelly P. A., Djiane J., Binder L., Gertler A. Mitogenic and binding properties of monoclonal antibodies to the prolactin receptor in Nb2 rat lymphoma cells. Selective enhancement by anti-mouse IgG. J Biol Chem. 1990 Sep 5;265(25):14770–14776. [PubMed] [Google Scholar]
- Hill S. M., Blask D. E. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988 Nov 1;48(21):6121–6126. [PubMed] [Google Scholar]
- Hill S. M., Spriggs L. L., Simon M. A., Muraoka H., Blask D. E. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Lett. 1992 Jul 10;64(3):249–256. doi: 10.1016/0304-3835(92)90050-6. [DOI] [PubMed] [Google Scholar]
- Jozan S., Tournier J. F., Tauber J. P., Bayard F. Adaptation of the human breast cancer cell line MCF-7 to serum free medium culture on extracellular matrix. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1566–1570. doi: 10.1016/s0006-291x(82)80178-2. [DOI] [PubMed] [Google Scholar]
- Kothari L. S., Shah P. N., Mhatre M. C. Pineal ablation in varying photoperiods and the incidence of 9,10-dimethyl-1,2-benzanthracene induced mammary cancer in rats. Cancer Lett. 1984 Feb;22(1):99–102. doi: 10.1016/0304-3835(84)90050-8. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Monaco M., Pinkus L., Engel L. Model systems for the study of estrogen action in tissue culture. J Steroid Biochem. 1976 Nov-Dec;7(11-12):1045–1051. doi: 10.1016/0022-4731(76)90032-7. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Murphy L. C., Vrhovsek E., Sutherland R. L., Lazarus L. Correlation of lactogenic receptor concentration in human breast cancer with estrogen receptor concentration. Cancer Res. 1984 May;44(5):1963–1968. [PubMed] [Google Scholar]
- Okamura H., Zachwieja J., Raguet S., Kelly P. A. Characterization and applications of monoclonal antibodies to the prolactin receptor. Endocrinology. 1989 May;124(5):2499–2508. doi: 10.1210/endo-124-5-2499. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991 May;12(2):151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980 Spring;1(2):109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Sanchez-Barcelo E. J., Mediavilla M. D., Tucker H. A. Influence of melatonin on mammary gland growth: in vivo and in vitro studies. Proc Soc Exp Biol Med. 1990 Jun;194(2):103–107. doi: 10.3181/00379727-194-43063. [DOI] [PubMed] [Google Scholar]
- Sanchez-Barcelo E. J., Mediavilla M. D., Zinn S. A., Buchanan B. A., Chapin L. T., Tucker H. A. Melatonin suppression of mammary growth in heifers. Biol Reprod. 1991 May;44(5):875–879. doi: 10.1095/biolreprod44.5.875. [DOI] [PubMed] [Google Scholar]
- Shafie S., Brooks S. C. Effect of prolactin on growth and the estrogen receptor level of human breast cancer cells (MCF-7). Cancer Res. 1977 Mar;37(3):792–799. [PubMed] [Google Scholar]
- Shah P. N., Mhatre M. C., Kothari L. S. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 1984 Aug;44(8):3403–3407. [PubMed] [Google Scholar]
- Shellard S. A., Whelan R. D., Hill B. T. Growth inhibitory and cytotoxic effects of melatonin and its metabolites on human tumour cell lines in vitro. Br J Cancer. 1989 Sep;60(3):288–290. doi: 10.1038/bjc.1989.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Murphy L. C., Tsuyuki D., Myal Y., Lee-Wing M., Iwasiow B. Biological actions of prolactin in human breast cancer. Recent Prog Horm Res. 1987;43:277–303. doi: 10.1016/b978-0-12-571143-2.50013-7. [DOI] [PubMed] [Google Scholar]
- Subramanian A., Kothari L. Suppressive effect by melatonin on different phases of 9,10-dimethyl-1,2-benzanthracene (DMBA)-induced rat mammary gland carcinogenesis. Anticancer Drugs. 1991 Jun;2(3):297–303. doi: 10.1097/00001813-199106000-00013. [DOI] [PubMed] [Google Scholar]
- Tamarkin L., Cohen M., Roselle D., Reichert C., Lippman M., Chabner B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res. 1981 Nov;41(11 Pt 1):4432–4436. [PubMed] [Google Scholar]
- Vonderhaar B. K. Estrogens are not required for prolactin induced growth of MCF-7 human breast cancer cells. Cancer Lett. 1989 Sep 15;47(1-2):105–110. doi: 10.1016/0304-3835(89)90184-5. [DOI] [PubMed] [Google Scholar]
- Welsch C. W. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985 Aug;45(8):3415–3443. [PubMed] [Google Scholar]


