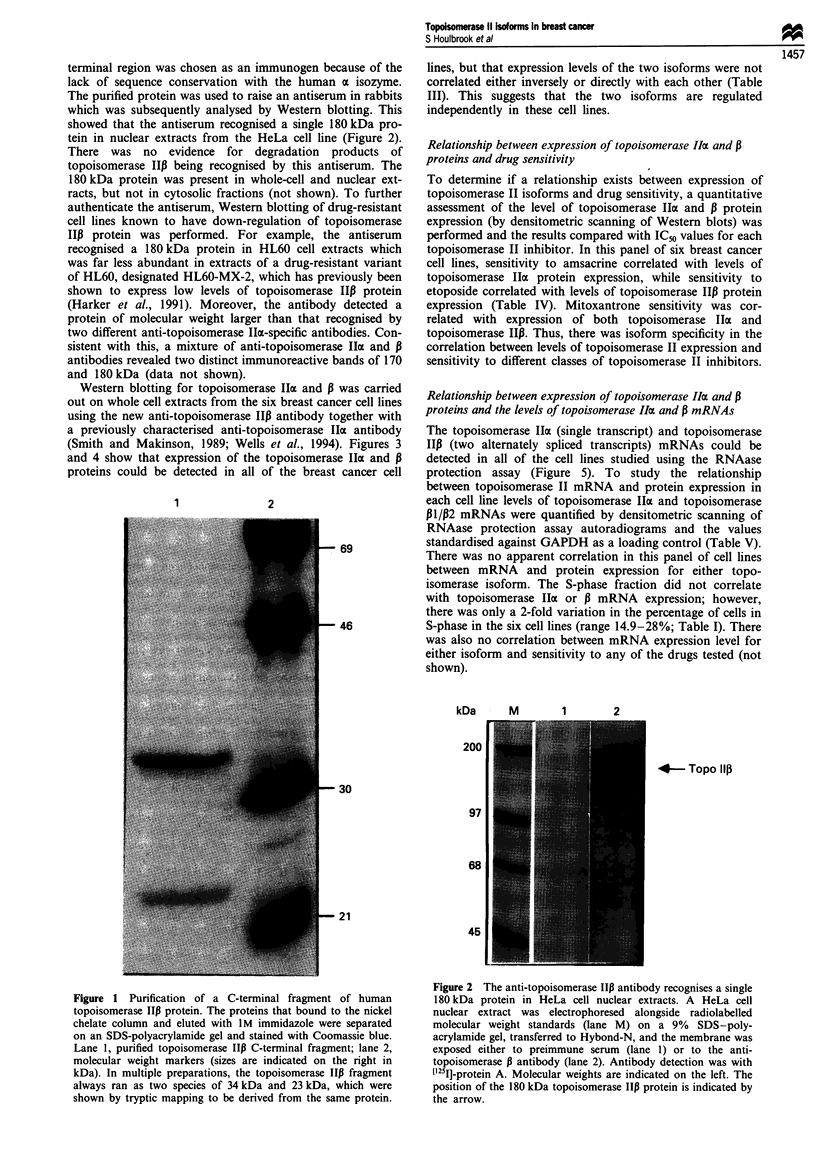
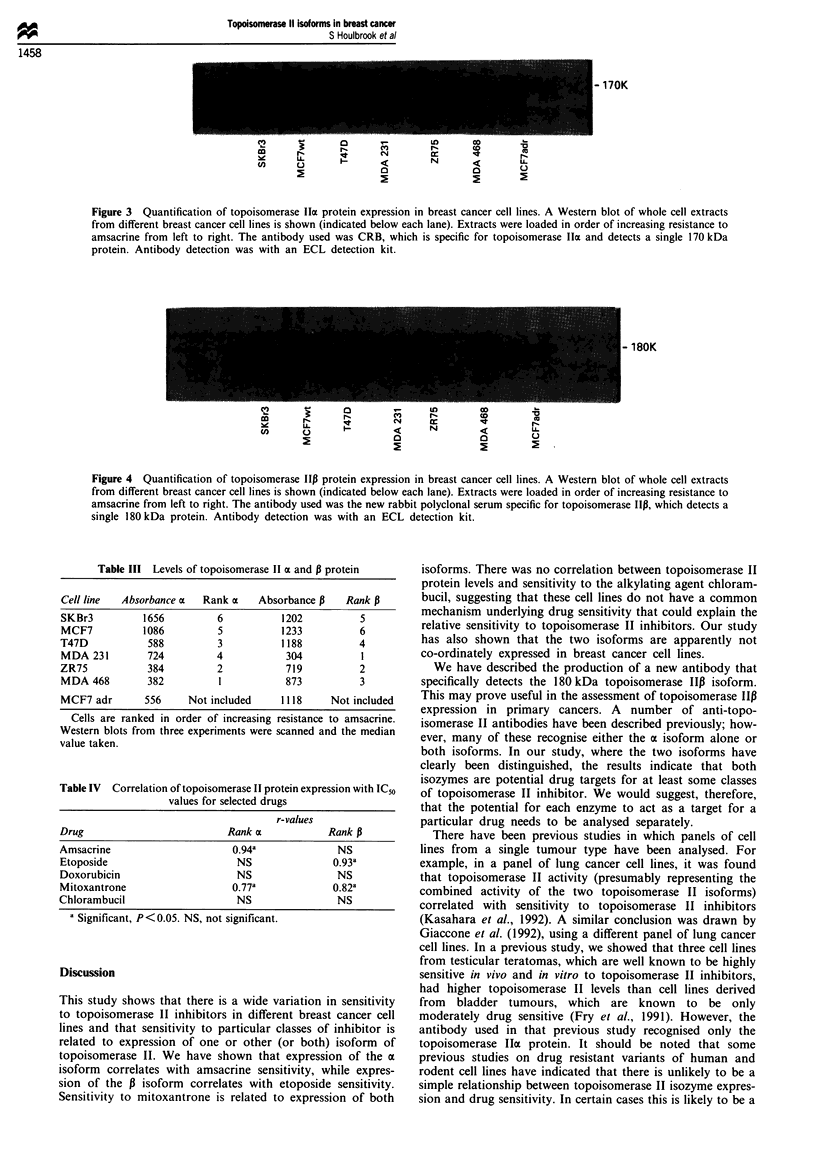
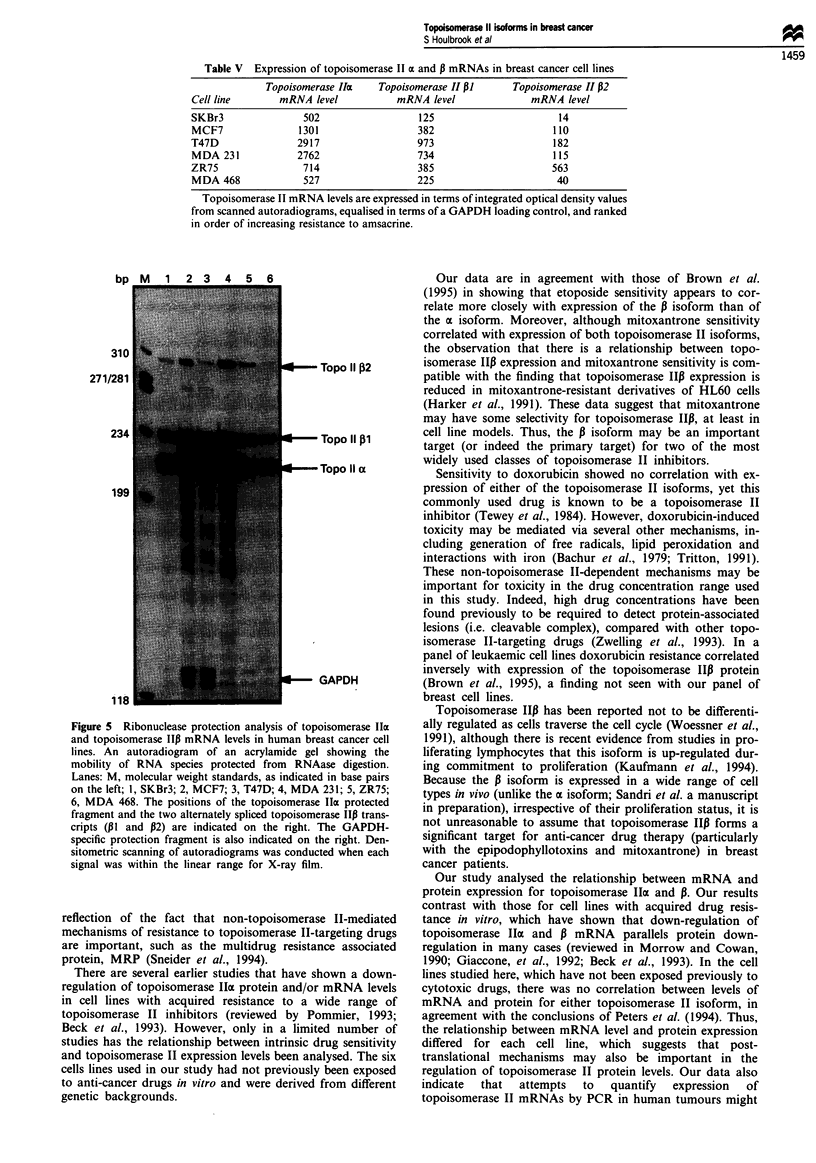
Abstract
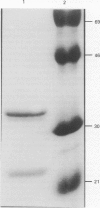
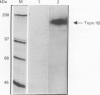
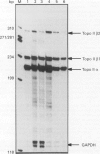
Topoisomerase II is a key target for many anti-cancer drugs used to treat breast cancer. In human cells there are two closely related, but differentially expressed, topoisomerase II isoforms, designated topoisomerase II alpha and beta. Here, we report the production of a new polyclonal antibody raised against a fragment of the C-terminal domain of the 180 kDa form of topoisomerase II (the beta isoform), which does not cross-react with the 170 kDa form (the alpha isoform). Using this antibody, together with a polyclonal antibody specific for the 170 kDa isoform of topoisomerase II, we have examined the relationship between the sensitivity of a panel of human breast cancer cell lines to different classes of topoisomerase II inhibitors and cellular levels of the topoisomerase II alpha and beta proteins. We found that sensitivity to amsacrine showed a correlation with the level of expression of topoisomerase II alpha protein, and that sensitivity to etoposide showed a similar correlation with the level of expression of topoisomerase II beta protein. There was also a relationship between sensitivity of these cell lines to mitoxantrone and the cellular level of both isoforms of topoisomerase II. No relationship was found between the level of mRNA for topoisomerase II alpha or beta, and either sensitivity of breast cancer cell lines to topoisomerase II inhibitors or the level of topoisomerase II protein expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin C. A., Fisher L. M. DNA topoisomerases: enzymes that change the shape of DNA. Sci Prog. 1990;74(294 Pt 2):147–161. [PubMed] [Google Scholar]
- Austin C. A., Sng J. H., Patel S., Fisher L. M. Novel HeLa topoisomerase II is the II beta isoform: complete coding sequence and homology with other type II topoisomerases. Biochim Biophys Acta. 1993 Mar 20;1172(3):283–291. doi: 10.1016/0167-4781(93)90215-y. [DOI] [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beck W. T., Cirtain M. C., Danks M. K., Felsted R. L., Safa A. R., Wolverton J. S., Suttle D. P., Trent J. M. Pharmacological, molecular, and cytogenetic analysis of "atypical" multidrug-resistant human leukemic cells. Cancer Res. 1987 Oct 15;47(20):5455–5460. [PubMed] [Google Scholar]
- Beck W. T., Danks M. K., Wolverton J. S., Kim R., Chen M. Drug resistance associated with altered DNA topoisomerase II. Adv Enzyme Regul. 1993;33:113–127. doi: 10.1016/0065-2571(93)90012-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley G., Ling V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994 Jun;13(2):223–233. doi: 10.1007/BF00689638. [DOI] [PubMed] [Google Scholar]
- Brown G. A., McPherson J. P., Gu L., Hedley D. W., Toso R., Deuchars K. L., Freedman M. H., Goldenberg G. J. Relationship of DNA topoisomerase II alpha and beta expression to cytotoxicity of antineoplastic agents in human acute lymphoblastic leukemia cell lines. Cancer Res. 1995 Jan 1;55(1):78–82. [PubMed] [Google Scholar]
- Capranico G., Zunino F. DNA topoisomerase-trapping antitumour drugs. Eur J Cancer. 1992;28A(12):2055–2060. doi: 10.1016/0959-8049(92)90255-z. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davies S. L., Jenkins J. R., Hickson I. D. Human cells express two differentially spliced forms of topoisomerase II beta mRNA. Nucleic Acids Res. 1993 Aug 11;21(16):3719–3723. doi: 10.1093/nar/21.16.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. M., Robson C. N., Davies S. L., Hickson I. D. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1988 Nov 25;263(33):17724–17729. [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Epstein R. J., Smith P. J. Estrogen-induced potentiation of DNA damage and cytotoxicity in human breast cancer cells treated with topoisomerase II-interactive antitumor drugs. Cancer Res. 1988 Jan 15;48(2):297–303. [PubMed] [Google Scholar]
- Epstein R. J., Smith P. J., Watson J. V., Bleehen N. M. Characterisation of VP-16-induced DNA cleavage in oestrogen-stimulated human breast cancer cells. Br J Cancer. 1988 May;57(5):445–450. doi: 10.1038/bjc.1988.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A. M., Chresta C. M., Davies S. M., Walker M. C., Harris A. L., Hartley J. A., Masters J. R., Hickson I. D. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991 Dec 15;51(24):6592–6595. [PubMed] [Google Scholar]
- Giaccone G., Gazdar A. F., Beck H., Zunino F., Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992 Apr 1;52(7):1666–1674. [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Harker W. G., Slade D. L., Drake F. H., Parr R. L. Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II beta isoform. Biochemistry. 1991 Oct 15;30(41):9953–9961. doi: 10.1021/bi00105a020. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Ayton P., Jones T., Davies S. L., Simmons D. L., Harris A. L., Sheer D., Hickson I. D. Isolation of cDNA clones encoding the beta isozyme of human DNA topoisomerase II and localisation of the gene to chromosome 3p24. Nucleic Acids Res. 1992 Nov 11;20(21):5587–5592. doi: 10.1093/nar/20.21.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S. A., Shields R. Hemodynamics of octreotide in hepatitis B-related cirrhosis. Gastroenterology. 1992 Dec;103(6):1992–1993. doi: 10.1016/0016-5085(92)91476-k. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Fujiwara Y., Sugimoto Y., Nishio K., Tamura T., Matsuda T., Saijo N. Determinants of response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst. 1992 Jan 15;84(2):113–118. doi: 10.1093/jnci/84.2.113. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Karp J. E., Jones R. J., Miller C. B., Schneider E., Zwelling L. A., Cowan K., Wendel K., Burke P. J. Topoisomerase II levels and drug sensitivity in adult acute myelogenous leukemia. Blood. 1994 Jan 15;83(2):517–530. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Morrow C. S., Cowan K. H. Multidrug resistance associated with altered topoisomerase II activity--topoisomerases II as targets for rational drug design. J Natl Cancer Inst. 1990 Apr 18;82(8):638–639. doi: 10.1093/jnci/82.8.638. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L., Gale K. C. Catalytic function of DNA topoisomerase II. Bioessays. 1991 Jun;13(6):269–273. doi: 10.1002/bies.950130603. [DOI] [PubMed] [Google Scholar]
- Patel S., Fisher L. M. Novel selection and genetic characterisation of an etoposide-resistant human leukaemic CCRF-CEM cell line. Br J Cancer. 1993 Mar;67(3):456–463. doi: 10.1038/bjc.1993.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. DNA topoisomerase I and II in cancer chemotherapy: update and perspectives. Cancer Chemother Pharmacol. 1993;32(2):103–108. doi: 10.1007/BF00685611. [DOI] [PubMed] [Google Scholar]
- Schneider E., Horton J. K., Yang C. H., Nakagawa M., Cowan K. H. Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 1994 Jan 1;54(1):152–158. [PubMed] [Google Scholar]
- Smith P. J., Makinson T. A. Cellular consequences of overproduction of DNA topoisomerase II in an ataxia-telangiectasia cell line. Cancer Res. 1989 Mar 1;49(5):1118–1124. [PubMed] [Google Scholar]
- Tan K. B., Dorman T. E., Falls K. M., Chung T. D., Mirabelli C. K., Crooke S. T., Mao J. Topoisomerase II alpha and topoisomerase II beta genes: characterization and mapping to human chromosomes 17 and 3, respectively. Cancer Res. 1992 Jan 1;52(1):231–234. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Tritton T. R. Cell surface actions of adriamycin. Pharmacol Ther. 1991;49(3):293–309. doi: 10.1016/0163-7258(91)90060-y. [DOI] [PubMed] [Google Scholar]
- Tsai-Pflugfelder M., Liu L. F., Liu A. A., Tewey K. M., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Wang J. C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt P. M., Hickson I. D. Structure and function of type II DNA topoisomerases. Biochem J. 1994 Nov 1;303(Pt 3):681–695. doi: 10.1042/bj3030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells N. J., Addison C. M., Fry A. M., Ganapathi R., Hickson I. D. Serine 1524 is a major site of phosphorylation on human topoisomerase II alpha protein in vivo and is a substrate for casein kinase II in vitro. J Biol Chem. 1994 Nov 25;269(47):29746–29751. [PubMed] [Google Scholar]
- Woessner R. D., Mattern M. R., Mirabelli C. K., Johnson R. K., Drake F. H. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991 Apr;2(4):209–214. [PubMed] [Google Scholar]
- Zwelling L. A., Bales E., Altschuler E., Mayes J. Circumvention of resistance by doxorubicin, but not by idarubicin, in a human leukemia cell line containing an intercalator-resistant form of topoisomerase II: evidence for a non-topoisomerase II-mediated mechanism of doxorubicin cytotoxicity. Biochem Pharmacol. 1993 Jan 26;45(2):516–520. doi: 10.1016/0006-2952(93)90091-a. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Lippman M. E. Protein-associated intercalator-induced DNA scission is enhanced by estrogen stimulation in human breast cancer cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6182–6186. doi: 10.1073/pnas.80.20.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]