Abstract
The effect of co-inoculation of basement membrane matrix, Matrigel and two human breast cancer cell lines, BT-474 and SK-BR-3, was tested in immune-deficient mice. Both cell lines strongly overexpress c-ErbB-2 protein, whereas only BT-474 is reported to be oestrogen receptor positive. Co-inoculation of Matrigel and BT-474 cells but not of Matrigel and SK-BR-3 cells resulted in tumour formation in bg-nu-xid mice. Oestrogen supplementation greatly enhanced tumorigenicity, but did not seem to be an absolute requirement. In vivo, BT-474 cells grow as a poorly differentiated adenocarcinoma with a doubling time of 9.4 +/- 1.1 days after inoculation into the neck region. A high proliferative activity appears to be compensated by a relatively high rate of cell loss, as BT-474 tumours contain many cells with the typical morphology of apoptotic cell death. Wild-type p53, known to participate in the induction of apoptosis, is absent from the tumours, whereas Bcl-2, known to inhibit apoptosis, is expressed at intermediate levels. BT-474 tumours tend to metastasise to the regional lymph nodes and are capable of forming micrometastatic lesions in the lung. Flow cytometrical analysis of DNA ploidy demonstrated no change in tumours compared with the cell line. Immunohistochemical and flow cytometrical detection of a number of hormone and growth factor receptors, transcription factors, cell adhesion molecules and proteins involved in proliferation and cell death demonstrated no major changes in ploidy and phenotype of tumours compared with the cell line. High expression of the cell-surface molecules c-ErbB-2 and episialin make it a potentially useful model for research in immune therapy.
Full text
PDF

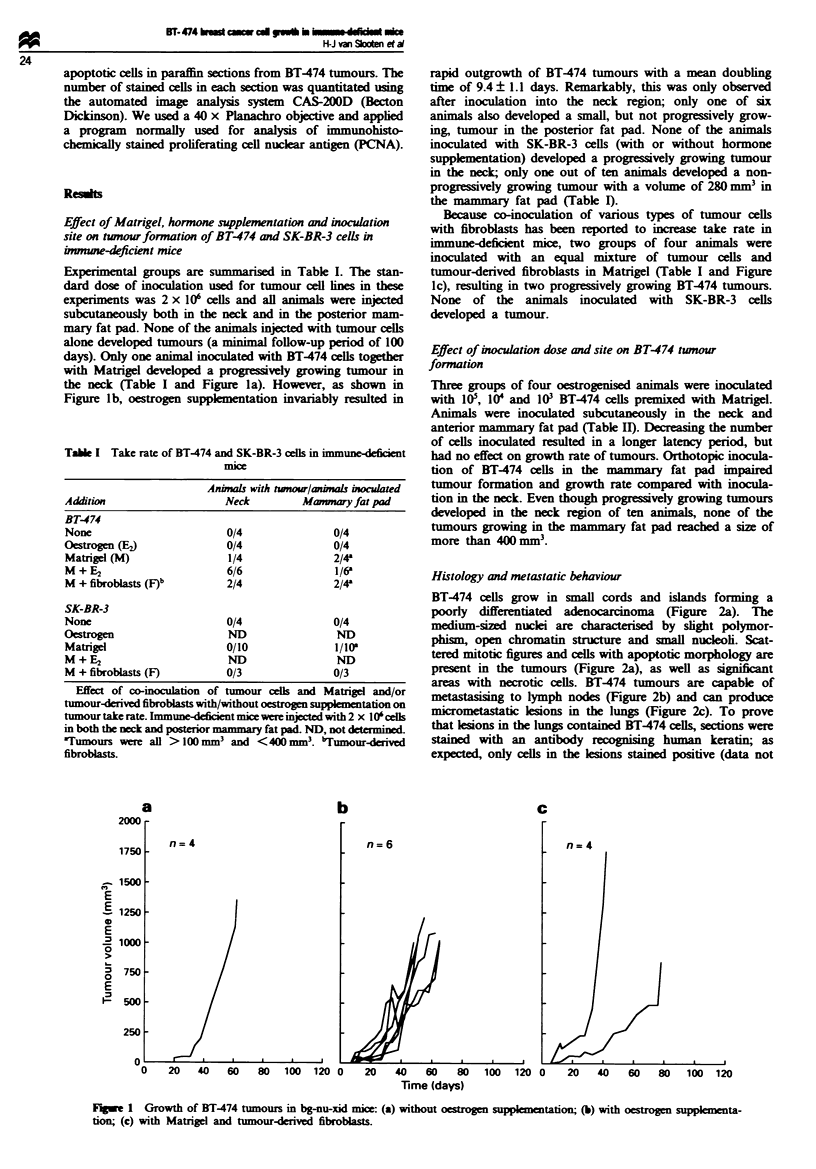
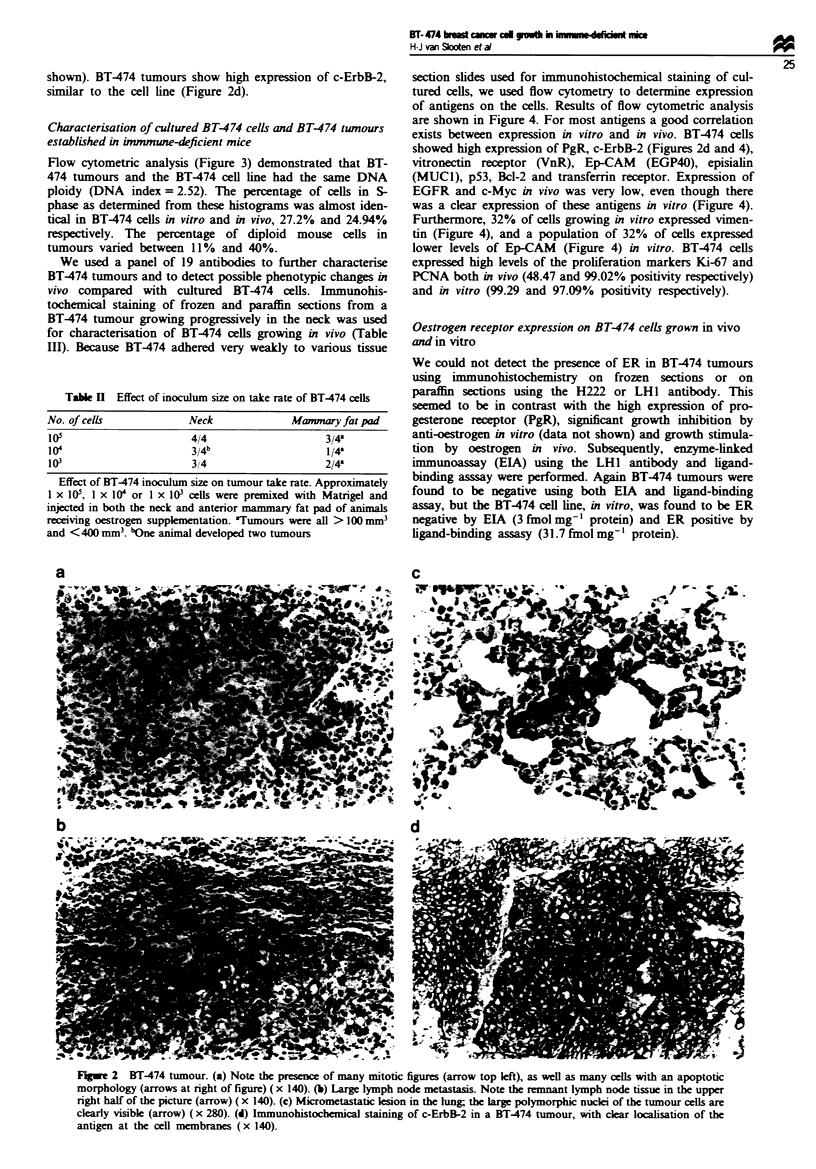
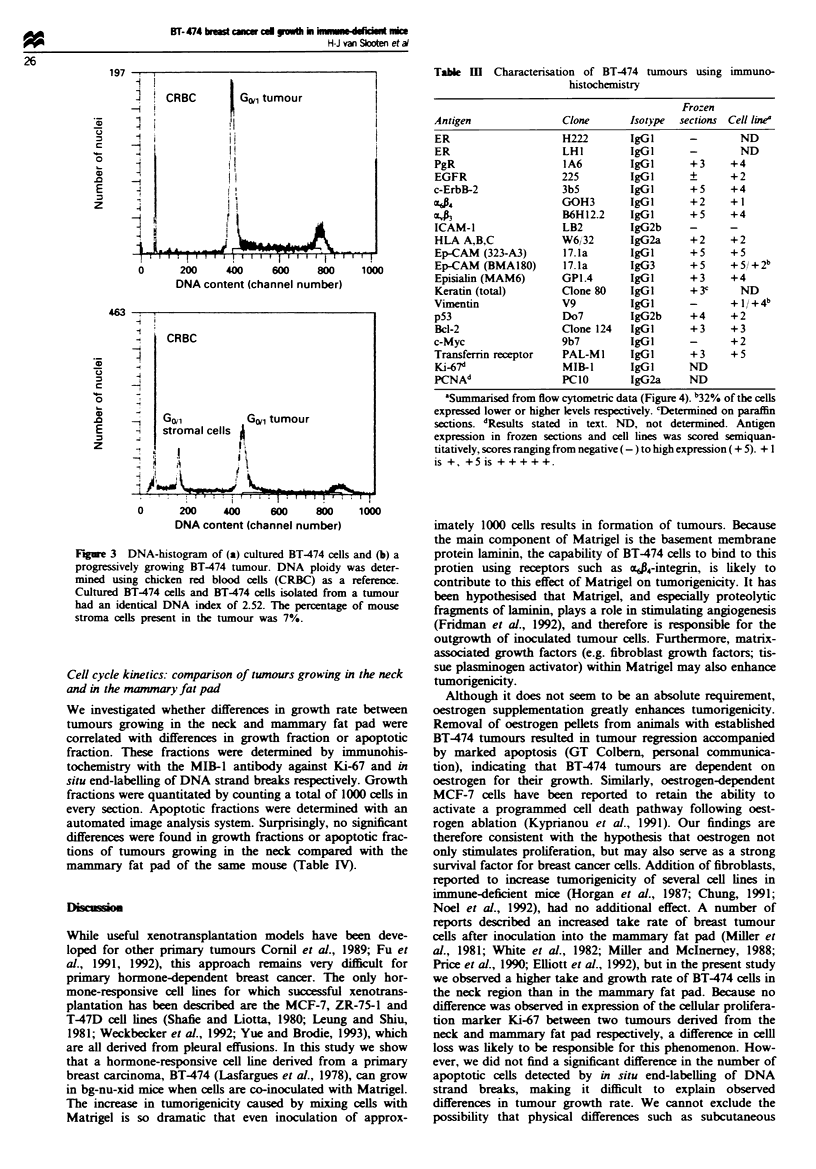
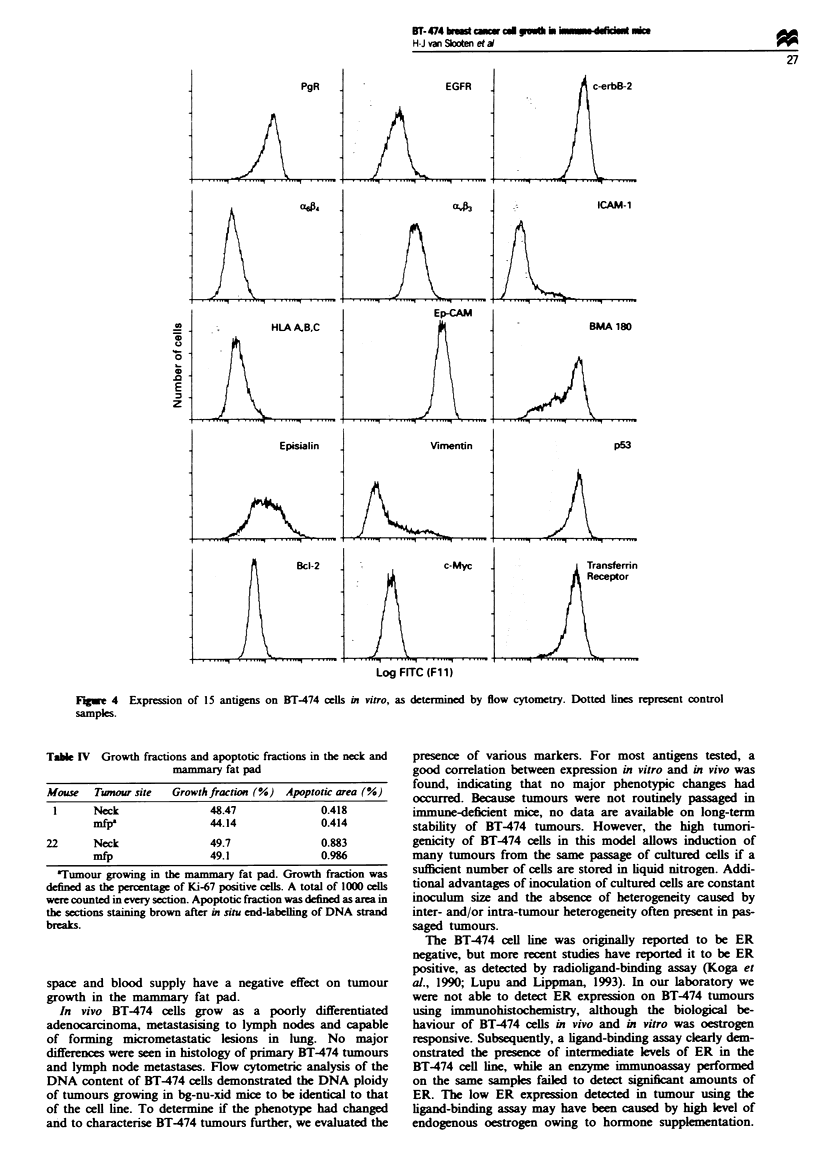



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Melchiori A., Garofalo A., Noonan D. M., Basolo F., Taraboletti G., Chader G. J., Gavazzi R. Matrigel promotes retinoblastoma cell growth in vitro and in vivo. Int J Cancer. 1992 Sep 9;52(2):234–240. doi: 10.1002/ijc.2910520214. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L., Kitten L. J., Coronado E. B., Jacobs S., Kull F. C., Jr, Allred D. C., Osborne C. K. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest. 1989 Nov;84(5):1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Matsumura Y., Baban D., Sun Y., Tarin D. Effects of inoculation site and Matrigel on growth and metastasis of human breast cancer cells. Br J Cancer. 1994 Aug;70(2):228–232. doi: 10.1038/bjc.1994.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Castles C. G., Fuqua S. A., Klotz D. M., Hill S. M. Expression of a constitutively active estrogen receptor variant in the estrogen receptor-negative BT-20 human breast cancer cell line. Cancer Res. 1993 Dec 15;53(24):5934–5939. [PubMed] [Google Scholar]
- Chung L. W. Fibroblasts are critical determinants in prostatic cancer growth and dissemination. Cancer Metastasis Rev. 1991 Oct;10(3):263–274. doi: 10.1007/BF00050797. [DOI] [PubMed] [Google Scholar]
- Cornil I., Man S., Fernandez B., Kerbel R. S. Enhanced tumorigenicity, melanogenesis, and metastases of a human malignant melanoma after subdermal implantation in nude mice. J Natl Cancer Inst. 1989 Jun 21;81(12):938–944. doi: 10.1093/jnci/81.12.938. [DOI] [PubMed] [Google Scholar]
- Corver W. E., Cornelisse C. J., Fleuren G. J. Simultaneous measurement of two cellular antigens and DNA using fluorescein-isothiocyanate, R-phycoerythrin, and propidium iodide on a standard FACScan. Cytometry. 1994 Feb 1;15(2):117–128. doi: 10.1002/cyto.990150205. [DOI] [PubMed] [Google Scholar]
- Dati C., Antoniotti S., Taverna D., Perroteau I., De Bortoli M. Inhibition of c-erbB-2 oncogene expression by estrogens in human breast cancer cells. Oncogene. 1990 Jul;5(7):1001–1006. [PubMed] [Google Scholar]
- Elledge R. M., McGuire W. L., Osborne C. K. Prognostic factors in breast cancer. Semin Oncol. 1992 Jun;19(3):244–253. [PubMed] [Google Scholar]
- Elliott B. E., Tam S. P., Dexter D., Chen Z. Q. Capacity of adipose tissue to promote growth and metastasis of a murine mammary carcinoma: effect of estrogen and progesterone. Int J Cancer. 1992 May 28;51(3):416–424. doi: 10.1002/ijc.2910510314. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Littlewood T. D. The role of c-myc in cell growth. Curr Opin Genet Dev. 1993 Feb;3(1):44–49. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Fridman R., Giaccone G., Kanemoto T., Martin G. R., Gazdar A. F., Mulshine J. L. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R., Kibbey M. C., Royce L. S., Zain M., Sweeney M., Jicha D. L., Yannelli J. R., Martin G. R., Kleinman H. K. Enhanced tumor growth of both primary and established human and murine tumor cells in athymic mice after coinjection with Matrigel. J Natl Cancer Inst. 1991 Jun 5;83(11):769–774. doi: 10.1093/jnci/83.11.769. [DOI] [PubMed] [Google Scholar]
- Fridman R., Sweeney T. M., Zain M., Martin G. R., Kleinman H. K. Malignant transformation of NIH-3T3 cells after subcutaneous co-injection with a reconstituted basement membrane (matrigel). Int J Cancer. 1992 Jul 9;51(5):740–744. doi: 10.1002/ijc.2910510513. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Besterman J. M., Monosov A., Hoffman R. M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Guadagni F., Hoffman R. M. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. G., Tilley W. D., De Young N. J., Lensink I. L., Dixon P. D., Horsfall D. J. Inhibition of T47D human breast cancer cell growth by the synthetic progestin R5020: effects of serum, estradiol, insulin, and EGF. Breast Cancer Res Treat. 1991 Dec;20(1):53–62. doi: 10.1007/BF01833357. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Jr, Williams L. J., Jr, Lee S. S., Shepard R. C. Heterotransplantation of human cancers into nude mice: a model system for human cancer chemotherapy. Cancer. 1978 Nov;42(5):2269–2281. doi: 10.1002/1097-0142(197811)42:5<2269::aid-cncr2820420527>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Hann S. R., Eisenman R. N. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984 Nov;4(11):2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth I. M., Wels W., Schlegel J., Müller M., Hynes N. E. Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br J Cancer. 1993 Dec;68(6):1140–1145. doi: 10.1038/bjc.1993.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Horgan K., Jones D. L., Mansel R. E. Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg. 1987 Mar;74(3):227–229. doi: 10.1002/bjs.1800740326. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. Mechanisms of hormone resistance in breast cancer. Breast Cancer Res Treat. 1993;26(2):119–130. doi: 10.1007/BF00689685. [DOI] [PubMed] [Google Scholar]
- Hudziak R. M., Lewis G. D., Shalaby M. R., Eessalu T. E., Aggarwal B. B., Ullrich A., Shepard H. M. Amplified expression of the HER2/ERBB2 oncogene induces resistance to tumor necrosis factor alpha in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5102–5106. doi: 10.1073/pnas.85.14.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E. Amplification and overexpression of the erbB-2 gene in human tumors: its involvement in tumor development, significance as a prognostic factor, and potential as a target for cancer therapy. Semin Cancer Biol. 1993 Feb;4(1):19–26. [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Nelson K. G., Bidwell M. C., Curtis S. W., Washburn T. F., McLachlan J. A., Korach K. S. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamesaki S., Kamesaki H., Jorgensen T. J., Tanizawa A., Pommier Y., Cossman J. bcl-2 protein inhibits etoposide-induced apoptosis through its effects on events subsequent to topoisomerase II-induced DNA strand breaks and their repair. Cancer Res. 1993 Sep 15;53(18):4251–4256. [PubMed] [Google Scholar]
- Koga M., Musgrove E. A., Sutherland R. L. Differential effects of phorbol ester on epidermal growth factor receptors in estrogen receptor-positive and -negative breast cancer cell lines. Cancer Res. 1990 Aug 15;50(16):4849–4855. [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Lasfargues E. Y., Coutinho W. G., Redfield E. S. Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst. 1978 Oct;61(4):967–978. [PubMed] [Google Scholar]
- Leung C. K., Shiu R. P. Required presence of both estrogen and pituitary factors for the growth of human breast cancer cells in athymic nude mice. Cancer Res. 1981 Feb;41(2):546–551. [PubMed] [Google Scholar]
- Lichtenstein A., Berenson J., Gera J. F., Waldburger K., Martinez-Maza O., Berek J. S. Resistance of human ovarian cancer cells to tumor necrosis factor and lymphokine-activated killer cells: correlation with expression of HER2/neu oncogenes. Cancer Res. 1990 Nov 15;50(22):7364–7370. [PubMed] [Google Scholar]
- Long B., McKibben B. M., Lynch M., van den Berg H. W. Changes in epidermal growth factor receptor expression and response to ligand associated with acquired tamoxifen resistance or oestrogen independence in the ZR-75-1 human breast cancer cell line. Br J Cancer. 1992 Jun;65(6):865–869. doi: 10.1038/bjc.1992.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lupu R., Lippman M. E. William L. McGuire Memorial Symposium. The role of erbB2 signal transduction pathways in human breast cancer. Breast Cancer Res Treat. 1993;27(1-2):83–93. doi: 10.1007/BF00683195. [DOI] [PubMed] [Google Scholar]
- Mehta R. R., Graves J. M., Hart G. D., Shilkaitis A., Das Gupta T. K. Growth and metastasis of human breast carcinomas with Matrigel in athymic mice. Breast Cancer Res Treat. 1993;25(1):65–71. doi: 10.1007/BF00662402. [DOI] [PubMed] [Google Scholar]
- Miller F. R., McInerney D. Epithelial component of host-tumor interactions in the orthotopic site preference of a mouse mammary tumor. Cancer Res. 1988 Jul 1;48(13):3698–3701. [PubMed] [Google Scholar]
- Miller F. R., Medina D., Heppner G. H. Preferential growth of mammary tumors in intact mammary fatpads. Cancer Res. 1981 Oct;41(10):3863–3867. [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Endogenous growth factor expression in T-47D, human breast cancer cells, associated with reduced sensitivity to antiproliferative effects of progestins and antiestrogens. Cancer Res. 1989 Feb 1;49(3):599–604. [PubMed] [Google Scholar]
- Niehans G. A., Singleton T. P., Dykoski D., Kiang D. T. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst. 1993 Aug 4;85(15):1230–1235. doi: 10.1093/jnci/85.15.1230. [DOI] [PubMed] [Google Scholar]
- Noel A., Simon N., Raus J., Foidart J. M. Basement membrane components (matrigel) promote the tumorigenicity of human breast adenocarcinoma MCF7 cells and provide an in vivo model to assess the responsiveness of cells to estrogen. Biochem Pharmacol. 1992 Mar 17;43(6):1263–1267. doi: 10.1016/0006-2952(92)90501-9. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Passaniti A., Isaacs J. T., Haney J. A., Adler S. W., Cujdik T. J., Long P. V., Kleinman H. K. Stimulation of human prostatic carcinoma tumor growth in athymic mice and control of migration in culture by extracellular matrix. Int J Cancer. 1992 May 8;51(2):318–324. doi: 10.1002/ijc.2910510224. [DOI] [PubMed] [Google Scholar]
- Pavelic Z. P., Pavelic L., Lower E. E., Gapany M., Gapany S., Barker E. A., Preisler H. D. c-myc, c-erbB-2, and Ki-67 expression in normal breast tissue and in invasive and noninvasive breast carcinoma. Cancer Res. 1992 May 1;52(9):2597–2602. [PubMed] [Google Scholar]
- Pretlow T. G., Delmoro C. M., Dilley G. G., Spadafora C. G., Pretlow T. P. Transplantation of human prostatic carcinoma into nude mice in Matrigel. Cancer Res. 1991 Jul 15;51(14):3814–3817. [PubMed] [Google Scholar]
- Price J. E., Polyzos A., Zhang R. D., Daniels L. M. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990 Feb 1;50(3):717–721. [PubMed] [Google Scholar]
- Read L. D., Keith D., Jr, Slamon D. J., Katzenellenbogen B. S. Hormonal modulation of HER-2/neu protooncogene messenger ribonucleic acid and p185 protein expression in human breast cancer cell lines. Cancer Res. 1990 Jul 1;50(13):3947–3951. [PubMed] [Google Scholar]
- Sebesteny A., Taylor-Papadimitriou J., Ceriani R., Millis R., Schmitt C., Trevan D. Primary human breast carcinomas transplantable in the nude mouse. J Natl Cancer Inst. 1979 Dec;63(6):1331–1337. [PubMed] [Google Scholar]
- Shafie S. M., Liotta L. A. Formation of metastasis by human breast carcinoma cells (MCF-7) in nude mice. Cancer Lett. 1980 Dec;11(2):81–87. doi: 10.1016/0304-3835(80)90097-x. [DOI] [PubMed] [Google Scholar]
- Sterling-Levis K., White L., Trickett A. E., Gramacho C., Pittman S. M., Tobias V. Heterotransplantation of early B-lineage acute lymphoblastic leukemia using a solubilized attachment matrix (Matrigel). Cancer Res. 1993 Mar 15;53(6):1222–1225. [PubMed] [Google Scholar]
- Topley P., Jenkins D. C., Jessup E. A., Stables J. N. Effect of reconstituted basement membrane components on the growth of a panel of human tumour cell lines in nude mice. Br J Cancer. 1993 May;67(5):953–958. doi: 10.1038/bjc.1993.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda R., Nakayama M., Heinen E., Miyake K., Suzuki K., Sugai N., Kojima M. Emperipolesis of lymphoid cells by human follicular dendritic cells in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(2):69–78. doi: 10.1007/BF02899667. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Weckbecker G., Liu R., Tolcsvai L., Bruns C. Antiproliferative effects of the somatostatin analogue octreotide (SMS 201-995) on ZR-75-1 human breast cancer cells in vivo and in vitro. Cancer Res. 1992 Sep 15;52(18):4973–4978. [PubMed] [Google Scholar]
- White A. C., Levy J. A., McGrath C. M. Site-selective growth of a hormone-responsive human breast carcinoma in athymic mice. Cancer Res. 1982 Mar;42(3):906–912. [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Wiltschke C., Tyl E., Speiser P., Steininger A., Zeillinger R., Kury F., Czerwenka K., Kubista E., Preis P., Krainer M. Increased natural killer cell activity correlates with low or negative expression of the HER-2/neu oncogene in patients with breast cancer. Cancer. 1994 Jan 1;73(1):135–139. doi: 10.1002/1097-0142(19940101)73:1<135::aid-cncr2820730123>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Wärri A. M., Laine A. M., Majasuo K. E., Alitalo K. K., Härkönen P. L. Estrogen suppression of erbB2 expression is associated with increased growth rate of ZR-75-1 human breast cancer cells in vitro and in nude mice. Int J Cancer. 1991 Oct 21;49(4):616–623. doi: 10.1002/ijc.2910490425. [DOI] [PubMed] [Google Scholar]
- Yue W., Brodie A. MCF-7 human breast carcinomas in nude mice as a model for evaluating aromatase inhibitors. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):671–673. doi: 10.1016/0960-0760(93)90278-5. [DOI] [PubMed] [Google Scholar]
- Zhu Y. M., Bradbury D. A., Russell N. H. Wild-type p53 is required for apoptosis induced by growth factor deprivation in factor-dependent leukaemic cells. Br J Cancer. 1994 Mar;69(3):468–472. doi: 10.1038/bjc.1994.85. [DOI] [PMC free article] [PubMed] [Google Scholar]



