Abstract
The sex pheromone of the female processionary moth, Thaumetopoea pityocampa, is a unique C16 enyne acetate that is biosynthesized from palmitic acid. Three consecutive desaturation reactions transform this saturated precursor into the triunsaturated fatty acyl intermediate: formation of (Z)-11-hexadecenoic acid, acetylenation to 11-hexadecynoic acid, and final Δ13 desaturation to (Z)-13-hexadecen-11-ynoic acid. By using degenerate primers common to all reported insect desaturases, a single cDNA sequence was isolated from total RNA of T. pityocampa female pheromone glands. The full-length transcript of this putative desaturase was expressed in elo1Δ/ole1Δ yeast mutants (both elongase 1 and Δ9 desaturase-deficient) for functional assays. The construct fully rescued the Δole1 yeast phenotype, confirming its desaturase activity. Analysis of the unsaturated products from transformed yeast extracts demonstrated that the cloned enzyme showed Δ11 desaturase, Δ11 acetylenase, and Δ13 desaturase activities. Therefore, this single desaturase may account for the three desaturation steps involved in the sex pheromone biosynthetic pathway of the processionary moth.
Keywords: acetylene, cloning, enyne, Thaumetopoea pityocampa, yeast
The ability of cells to modulate the degree of unsaturation of their membranes is mainly determined by the action of acyl-CoA desaturases, which catalyze the introduction of unsaturations into long-chain fatty acids (1). These enzymes are crucial in regular lipid metabolism and also contribute to the cellular response to changes in environmental temperatures (2). The desaturases expressed in female sex-pheromone glands of different moth species have evolved to play a key role in the biosynthesis of sex pheromones, exhibiting an amazing diversity of substrate and regio- and stereospecifities. Unlike the metabolic Δ9 acyl-CoA desaturases, pheromone gland desaturases catalyze the formation of uncommon unsaturated fatty acyl-CoA esters with variable chain lengths, different locations of unsaturations, and either the ordinary Z or the unusual E double bond geometry (3–10).
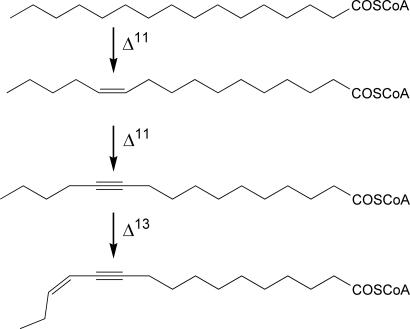
Females of the processionary moth Thaumetopoea pityocampa use a single chemical as a sex pheromone for mate attraction, (Z)-13-hexadecen-11-ynyl acetate (11). This circumstance is uncommon among lepidopteran species, which frequently use blends of compounds as sex pheromones (12). Probably, the unique structure of T. pityocampa, with a combination of double and triple bonds, prevents cross-talk between closely related species and makes unnecessary the fine tuning generally provided by minor components in other moth species. Three different desaturation reactions are involved in the biosynthetic pathway of this semiochemical from palmitic acid. The initial Δ11 desaturation gives rise to (Z)-11-hexadecenoic acid, which is then desaturated to 11-hexadecynoic acid. This compound is further Δ13 desaturated to give the enynoic immediate pheromone precursor (Fig. 1), which is finally reduced and acetylated (13–17).
Fig. 1.
Desaturation reactions involved in the biosynthetic pathway of T. pityocampa sex pheromone. Δ11, Δ11 desaturation or acetylenation; Δ13, Δ13 desaturation.
Cloned desaturases specifically involved in sex pheromone biosynthesis with unusual regiospecificities include the Δ14 desaturase of Ostrinia species (18), the Δ10 desaturase of Planotortrix octo (3), and the bifunctional desaturases of Bombyx mori (9) and Spodoptera littoralis (10, 19). Because of the unique chemical structure of its sex pheromone, cloning the acyl-CoA desaturases from T. pityocampa pheromone gland might uncover new desaturases with activities, like Δ11 acetylenase and Δ13 desaturase, so far unreported in the Animal Kingdom. Desaturases able to insert a triple bond (acetylenases) in fatty acid chains have been up to now characterized in the moss Ceratodon purpureus (20) and in the plant Crepis alpina (Compositae) (21). In this article, we report on the cloning and functional expression of a single multifunctional desaturase from the T. pityocampa pheromone gland that exhibits all of the required activities to biosynthesize the sex pheromone chemical backbone from palmitic acid.
Results
Cloning of the Pheromone Gland Fatty-Acyl Desaturases.
Total RNA was isolated from some 200 pheromone glands of T. pityocampa females. A 550-bp DNA fragment was amplified by PCR using degenerate primers common to all insect desaturases, as described (22). This fragment encompasses the central conserved region of the enzyme, including the key His rich domains essential for the catalytic activity and conserved in all of the fatty acid desaturases cloned so far (3–5, 23, 24). A total of 10 independent PCR fragments (4 independent RNA preparations) were cloned and sequenced. All clones encompassed an almost identical DNA sequence, with <1% divergence, attributable to PCR errors. Therefore, we concluded that a single transcript accounts for most, if not all, the desaturase gene expression in T. pityocampa female pheromone glands.
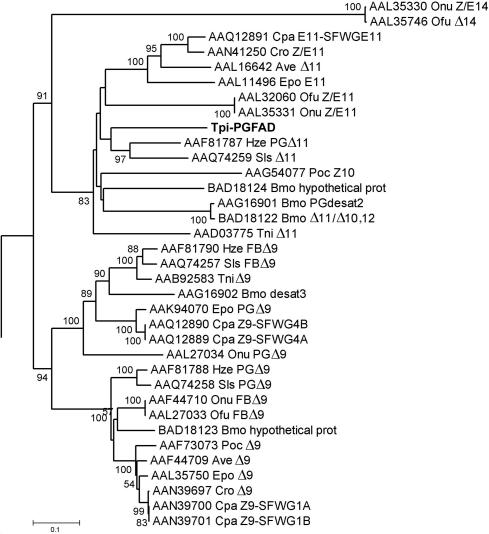
From the sequence of the central domain, we cloned the full-length cDNA. This putative desaturase transcript spanned 1,191 bp and encompassed an ORF of 1,041 bp, corresponding to a protein with 347 aa (Fig. 2). The deduced aa sequence showed high homology with various known insect Δ11 desaturases (3–9, 24) (Fig. 2). A phylogenetic analysis indicated a high amino acid sequence similarity between the putative T. pityocampa pheromone gland desaturase and several Δ10, Δ11, and bifunctional desaturases from closely related lepidopteran species (Fig. 3). The predicted protein shares several key features with other known insect desaturases, like the position and length of the transmembrane stretches relative to the conserved His boxes and the presence of endoplasmic reticulum membrane retention signals in the C terminus (Fig. 2). Therefore, sequence analyses strongly suggested that the isolated clone encompassed an authentic fatty acid desaturase, which was named Tpi-PGFAD (T. pityocampa pheromone gland fatty acyl desaturase).
Fig. 2.
Alignment of the predicted protein isolated from T. pityocampa pheromone glands. The alignment was performed with reported sequences (3–9, 24) with the Clustal 1.8 program. The transmembrane domains (http://bioinfo4.limbo.ifm.liu.se/tmap/) are shown with black lines above the sequence, and the endoplasmic reticulum retention signal as proposed by PSORTII tool (www.ExPASy.org) is in bold and underlined (amino acids 289–292). The three His domains are indicated with a “His box” label.
Fig. 3.
Phylogenetic tree of lepidopteran desaturases including the Tpi-PGFAD. The computer program MEGA2 (43) was used to reconstruct the tree from deduced amino acid sequences by using the neighbor-joining method (44). Numbers along branches indicate bootstrap support from 1,500 replicates. The protein accession numbers are given before the species abbreviations (Ave, Argyrotaenia velutinana; Bmo, B. mori; Cpa, Choristoneura parallela; Cpo, Cydia pomonella; Cro, Choristoneura rosaceana; Epo, Epiphyas postvittana; Hze, Helicoverpa zea; Ofu, O. furnacalis; Onu, O. nubilalis; Poc, P. octo; Sls, S. littoralis; Tni, T. ni; Tpi, T. pityocampa). The tree is rooted on the tick stearoylCoA desaturase for comparison with previous studies (18, 41).
Protein Expression.
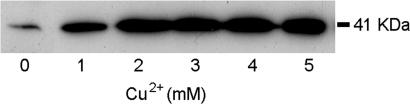
The putative Tpi-PGFAD gene was cloned into the pYEXTHS-BN system (25) for expression in a desaturase- and elongase-deficient mutant elo1Δ/ole1Δ yeast strain (26) (strain YMS1). Tpi-PGFAD expression was evaluated in lysates from YMS1 cells incubated with different concentrations of copper sulfate by SDS/PAGE and immunoblotting by using a His6 antibody (25). The analyses revealed copper-dependent expression of a 40-kDa immunoreactive protein, in agreement with the expected molecular weight for the recombinant desaturase with the hexahistidine tagTpi-PGFAD expression pattern in YMS1 cells agreed with the expected regulation of the vector pCUP1 promoter by Cu2+ salts, showing maximal expression at ≈2 mM Cu2+, and very low expression in the absence of Cu2+ (Fig. 4).
Fig. 4.
Western blot analysis of proteins from pYEXTHS-BN-Tpi-PGFAD transformed yeast grown in the presence of different CuSO4 concentrations. Proteins were extracted from cell lysates and separated by SDS/PAGE. Fifteen micrograms of protein were loaded in each lane. The hexahistidine-tagged protein was detected by immunoblotting with an anti-His6 antibody. The size of the molecular mass marker is indicated on the right.
Functional Assay of Tpi-PGFAD.
Unlike the parental strain, YMS1 cells were able to grow in the absence of exogenous unsaturated fatty acids, but only in the presence of Cu2+. This functional complementation of the ole1Δ phenotype by Tpi-PGFAD demonstrates that the cloned sequence truly encompassed a fatty acid desaturase, and that this enzyme was functional in yeast.
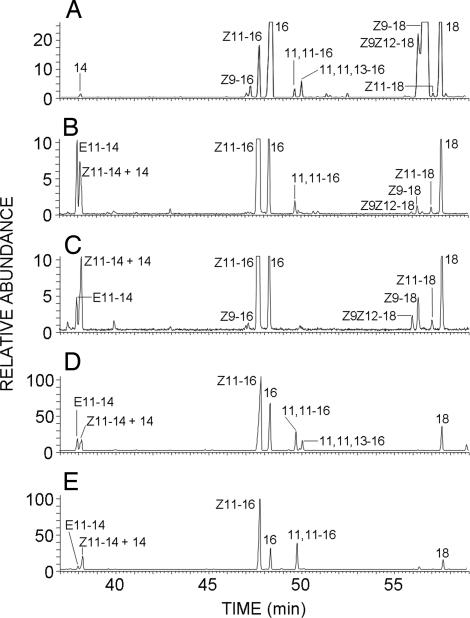
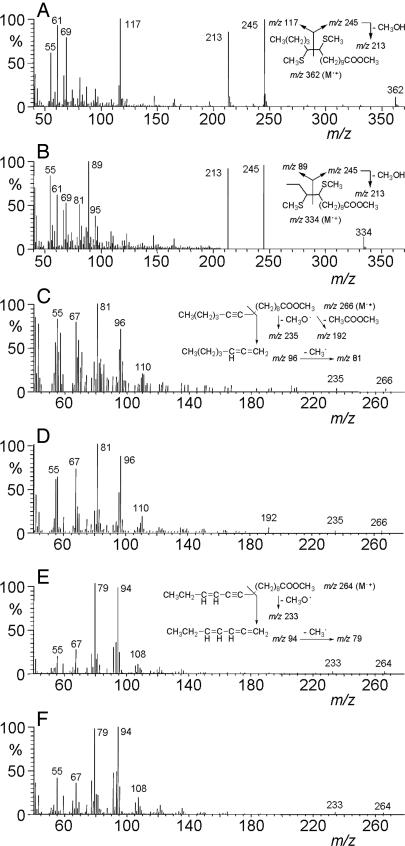
To characterize the desaturase activity, lipid extracts of copper-treated YMS1 cells were base methanolyzed and analyzed by GC/MS (gas chromatography coupled to mass spectrometry). A fatty acid methyl ester extract from T. pytiocampa pheromone glands was also prepared and analyzed to serve as a reference for comparison purposes. In this last case, GC/MS analysis showed the presence of the reported biosynthetic intermediates (13), namely (Z)-11-hexadecenoic acid, 11-hexadecynoic acid and (Z)-13-hexadecen-11-ynoic acid, in addition to other fatty acids common to cell membranes (Fig. 5A). YMS1 extracts contained large amounts of (Z)-11-hexadecenoic acid as well as smaller proportions of (Z) and (E)-11-tetradecenoic acid, in an 81:5:14 ratio, respectively (Fig. 5B). The double bond position in C11 was confirmed with dimethyl disulfide derivatization, which produced adducts showing the characteristic ions of methyl 11,12-bis(methylthio) C14 and C16 fatty esters (27) (Fig. 6 A and B). The presence of low amounts (1.3–2.1%) of methyl 11-hexadecynoate was also observed in the extracts (Fig. 5B), as concluded from the identity of both retention times (49.7 min) and mass spectra (Fig. 6 C and D) between the natural compound and an authentic synthetic standard. This compound was not produced in cells transformed with the S. littoralis Δ11 desaturase, which did produce the previously reported (10, 19) unsaturated intermediates (Fig. 5C).
Fig. 5.
GC/MS analysis of lipid extracts from yeast transformed with desaturase genes. Methanolyzed lipid extracts from T. pytiocampa pheromone glands (A) and yeast transformed with either pYEXTHS-BN-Tpi-PGFAD (B and D) or pYEXTHS-BN-SlsZ/E11 (S. littoralis pheromone gland Δ11/Δ10,12 desaturase) (C and E) were analyzed. Traces correspond to the ion current obtained by extraction of the molecular ions of methyl tetradecanoate (m/z 242), methyl tetradecenoates (m/z 240), methyl tetradecadienoates (m/z 238), methyl hexadecanoate (m/z 270), methyl hexadecenoates (m/z 268), methyl hexadecadienoates (m/z 266), methyl octadecanoate (m/z 298), methyl octadecenoates (m/z 298), methyl octadecadienoates (m/z 296), and the diagnostic ions of methyl 11-hexadecynoate (m/z 96) and methyl (Z)-13-hexadecen-11-ynoate (m/z 94). The mass spectra of compounds labeled as 11,11–16 in trace B (retention time 49.7 min) and 11,11,13–16 in trace D (retention time 50.0 min) are shown in Fig. 6 C and E, respectively, and those of their corresponding synthetic standards are depicted in Fig. 6 D and F. Transformants were grown in the presence of 2 mM CuSO4 with (D and E) or without (B and C) 0.5 mM 11-hexadecynoic acid. 14, methyl tetradecanoate; E11–14, methyl (E)-11-tetradecenoate; Z11–14, methyl (Z)-11-tetradecenoate; 16, methyl hexadecanoate; Z9–16, methyl (Z)–9–hexadecenoate; Z11–16, methyl (Z)–11–hexadecenoate; 11,11–16, methyl 11-hexadecynoate; 11,11,13–16:Me, methyl (Z)-13-hexadecen-11-ynoate; 18, methyl octadecanoate; Z9–18, methyl (Z)–9–octadecenoate; Z11–18, methyl (Z)–11–octadecenoate; Z9Z12–18, methyl (Z,Z)-9,12-octadecadienoate.
Fig. 6.
Mass spectra of selected fatty acid methyl esters. (A) Dimethyl disulfide adduct of methyl (Z)–11–hexadecenoate. (B) Dimethyl disulfide adduct of methyl (E)–11–tetradecenoate. (C) Natural methyl 11-hexadecynoate. (D) Synthetic methyl 11-hexadecynoate standard. (E) Natural methyl (Z)-13-hexadecen-11-ynoate. (F) Synthetic methyl (Z)-13-hexadecen-11-ynoate standard. The spectra of C and E correspond to peaks labeled as 11,11–16 and 11,11,13–16 in Fig. 5 B and D, respectively. The spectra of A and B correspond to the dimethyl disulfide adducts obtained from the fatty acid methyl ester mixture of Fig. 5B. The mass spectrum of the dimethyl disulfide derivative of methyl (Z)-11-tetradecenoate was identical to that of the E isomer and it is not shown. Retention times of dimethyl disulfide adducts were 31.9 min (methyl (Z)–11–tetradecenoate), 32.15 min (methyl (E)–11–tetradecenoate), and 33.94 min (methyl (Z)–11–hexadecenoate) (see Materials and Methods for chromatographic conditions).
Incubation of Tpi-PGFAD-expressing YMS1 cells with 11-hexadecynoic acid (0.5 mM) resulted in the production of low levels (3–5%) of a triunsaturated C16 fatty acid (Fig. 5D). This compound was identified as (Z)-13-hexadecen-11-ynoic acid on the basis of the GC/MS retention time (49.9 min) and mass spectrum (Fig. 6E) of its methyl ester, which were identical to those of an authentic synthetic sample (Fig. 6F). The mass spectrum included fragments at m/z 264 (M•+), 233 (M•+-31, loss of MeO•) and intense ions at m/z 79 and 94 (see Fig. 6E). Although the enyne was barely detected in transformants grown in the absence of added precursor, methyl (Z)-13-hexadecen-11-ynoate was consistently found in extracts from cells incubated in the presence of 11-hexadecynoic acid. Yeast strains expressing the S. littoralis (Fig. 5 C and E) or the Trichoplusia ni (data not shown) Δ11desaturase genes inserted in the same vector and cultured under the same conditions as YMS1 showed no detectable production of either 11-hexadecynoic acid or (Z)-13-hexadecen-11-ynoic acid, demonstrating that the Tpi-PGFAD gene was absolutely required for yeast to produce these compounds. Therefore, we concluded that the desaturase gene isolated from T. pityocampa pheromone glands encompasses a multifunctional enzyme with Δ11desaturase, Δ11acetylenase, and Δ13desaturase activities.
Discussion
The processionary moth sex pheromone, (Z)-13-hexadecen-11-ynyl acetate, is biosynthesized from palmitic acid by sequential Δ11 desaturation, Δ11 acetylenation and Δ13 desaturation, followed by final reduction and acetylation (13–17). This biosynthetic pathway suggested that three different desaturases were involved in the process. The isolation of a single putative desaturase gene from multiple candidate clones from female T. pityocampa pheromone glands indicated that all three reactions could be carried out by a single enzyme. This possibility was confirmed by functional assays, showing that Tpi-PGFAD was indeed able to catalyze each one of the desaturation reactions required for the biosynthesis of T. pityocampa sex pheromone.
Whereas multifunctional desaturases able to introduce double and triple bonds were unknown in the animal kingdom, some examples have been reported in plants. The Δ12 acetylenase of C. alpina produces a mixture of both (Z,Z) and (Z,E)-9,12-octadecadienoate from oleate, as well as (Z)-9-octadecen-12-ynoate from linoleate (21, 28, 29). Likewise, another bifunctional desaturase/acetylenase enzyme has been reported in the moss C. purpureus (20, 29). Bifunctional desaturases are not unusual in insects, as exemplified by the Δ11/Δ10,12 desaturases of B. mori (9) and S. littoralis (19). However, the case of the Tpi-PGFAD is unique because it exhibits not only dual Δ11/Δ13 desaturase activity, but also acetylenase activity, thus constituting a remarkable example of evolution of complex enzymatic activities to generate natural products in the most economical manner for the cell. Focusing on its Δ11 desaturase activity, Tpi-PGFAD main products are (Z)-11-hexadecenoic acid and both (Z)- and (E)-11-tetradecenoic acids, whereas (Z)-11-octadecenoic acid is produced in very low amounts (Fig. 5B). These results are similar to those found with the Δ11 desaturase of S. littoralis (Fig. 5H), which produced the C11-monounsaturated hexa- and tetradecenoates, but did not make the corresponding C18 compound when expressed in yeast by using the pYEXTHS-BN vector (19). In contrast, (Z)-11-octadecenoic acid is synthesized by yeast transformed with either pYEXTHS-BN or the YEpOLEX-based plasmids containing the T. ni Δ11 desaturase gene (5, 19). These differences suggest subtle dissimilarities in the tridimensional structure of the enzyme active sites, which result in different capabilities for accommodating long acyl substrates.
Besides its Δ11 desaturase activity, the Tpi-PGFAD showed also Δ13 desaturase activity when expressed in yeast. YMS1 cells produced very small amounts of acetylenic acid precursor, therefore, exogenous addition of 11-hexadecynoic acid was necessary for producing the enynoic acid at detectable levels. Attempts to increase the enyne production by augmenting the acetylene concentration in the culture medium resulted in cell growth inhibition. This apparent cytotoxicity is in accordance with reported antifungal activities of some acetylenic fatty acids of plant origin (30, 31) and may be explained considering the reported effects of lipids with acetylenic acyl groups on membrane biophysical properties, such as variations in phase transition temperatures (32–34). As a result, lipid fluidity of cell membranes may change beyond a point critical for normal cell growth and proliferation.
Several Δ9 desaturases have been cloned from moth pheromone glands and functionally expressed in yeast (6, 8, 10, 18, 24). However, none of the multiple clones from T. pityocampa pheromone glands analyzed corresponded to a Δ9 desaturase. This result is at variance with the occurrence of substantial amounts of oleic acid in the gland (see Fig. 5A). Oleic acid production requires Δ9 desaturase activity; its presence in the pheromone gland can be explained either by its being imported from surrounding tissues, or by its accumulation in the larval stage before moth eclosion.
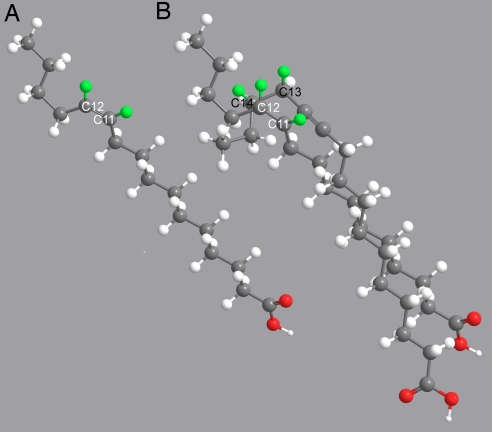
The coexistence of Δ11/Δ13 desaturase and acetylenase activities in a single multifunctional catalytic site can be rationalized on the basis of previously obtained mechanistic data. Experiments with mass labeled substrates demonstrated that all of the three reactions performed by Tpi-PGFAD begin with oxidation of the carbon atom located nearest the carboxylate end: C11 in Δ11 desaturation and acetylenation (35, 36) and C13 in Δ13 desaturation (37). Because a triple bond is shorter (1.21 Å) than a single bond (1.55 Å), accommodation of the Δ11 desaturase substrates in the enzyme active site sets C11 accessible to the iron oxidating function, whereas accommodation of 11-hexadecynoic acid leaves C13 better positioned for initial oxidation. These structural constraints become evident in molecular models, which show that C11 in both palmitic and (Z)-11-hexadecenoic acids, and C13 in 11-hexadecynoic acid are spatially close (Fig. 7). Confirmation of this proposal will require the structural resolution of fungal or animal membrane-bound desaturases because the soluble plant desaturase/acetylenase enzymes are phylogenetically unrelated and, therefore, structural information from them cannot be applied to the insect enzymes (38).
Fig. 7.
Molecular models of (Z)-11-hexadecenoic acid (A) and superimposed hexadecanoic and 11-hexadecynoic acids (B). In B, the two structures were overlayed after minimization of the C1-C10 chain with the Chem3D Ultra 9.0 program. For the sake of clarity, because palmitic and (Z)-11-hexadecenoic acid structures are almost completely superimposable, the latter is shown out of the overlayed structures. Carbon and oxygen atoms are depicted in dark gray and red, respectively. Hydrogens are shown in light gray, except for the hydrogen atoms removed from C11, C12, C13, and C14, which are colored in green (pro-(R) in the case of enantiotopic hydrogens). White-labeled C11 and C12 correspond to hexadecanoic and (Z)-11-hexadecenoic acid; black-labeled C13 and C14 correspond to 11-hexadecynoic acid.
Phylogenetic analysis of desaturases in Lepidoptera indicates that the T. pityocampa pheromone gland desaturase can be included within a branch containing Δ10 and Δ11 desaturases, as well as the bifunctional Δ11/Δ10,12 desaturases of B. mori and S. littoralis (Fig. 3). This partitioning suggests that this branch includes desaturases with regiospecificites ranging from Δ10 to Δ13. It would be interesting to clone and determine whether moth Δ12 desaturases, such as those involved in the biosynthesis of the Lymantria dispar (39), Cadra cautella (40), and S. exigua (40) sex pheromones, cluster also within this group. As more taxa are added to this branch with the cloning of new desaturase genes, in silico approaches suggested by Roelofs and Rooney (41) will probably allow prediction of the detailed function of presumed desaturase genes.
Materials and Methods
Collection of Insect Tissue, RNA Extraction, and Preparation of Fatty Acid Methyl Ester Extracts.
T. pityocampa female pupae were collected in the field and supplied by the Instituto para la Conservación de la Naturaleza (ICONA, Madrid, Spain). They were maintained at 25 ± 2°C in a 16-h:8-h light:dark photoperiod. Pheromone glands were carefully dissected from 1- to 2-day-old virgin female moths and stored at −80°C. Total RNA was isolated and purified from pheromone glands by using a total RNAqueous-4PCR Kit (Ambion, Valencia, CA) according to the procedures recommended by the manufacturer. Fatty acid methyl esters were obtained from freshly dissected glands as reported elsewhere (13).
Cloning and Sequence Analysis of Tpi-PGFAD.
Total RNA (5 ng, 20 ng, and 50 ng) was used to amplify the central region of the desaturase gene. Six degenerate primers described previously were used in OneStep RT-PCR (Qiagen, Austin, TX) following the manufacturer's instructions. The OneStep RT-PCR conditions were as follows: hot start at 50°C; reverse transcription at 50°C for 30 min; PCR activation at 95°C for 15 min, followed by 35 cycles using 94°C for 30 s, 50°C for 30 s (primers 5′-GGYATYACVGCHGGNGCWCA-3′ plus 5′-TGRTARTTRTGGAABSCYTCNCC-3′), or 45°C for 30 s (primers 5′-ATCACNGCHGGBGMYCAYMG-3′ plus 5′-GGRAASDYRTGRTGRWARTT-3′ and 5′-GACCAYMGNHWSCAYCA-3′ plus 5′-GGRWAVRYRTGRTGRTARTT-3′); 72°C for 1 min. A final step at 72°C for 10 min was performed to ensure completion of all extension reactions.
Amplification products were analyzed by electrophoresis in agarose gels. Specific amplification products were excised and eluted from gels, ligated to pTZ57R/T vector, and cloned into E. coli DH5α competent cells (Invitrogen, Burlington, ON, Canada) by using an InsT/Aclone PCR Product Cloning Kit (Fermentas, Vilnius, Lithuania) for sequencing in an Applied Biosystems 3730 DNA Analyzer.
A single fragment was obtained of ≈550 bp, which was homologous to the central region of other insect desaturases.
Based on this sequence information, specific primers were designed to obtain 3′ and 5′ ends by rapid amplification of cDNA ends. For the amplification of the 3′ end, we used the OneStep RT-PCR Kit (Qiagen) using sequence-specific (5′-GCCTGGCACATCTGTATACTTCG-3′, 5′-GGACGAGCAGCCACCCGACAT-3′, and 5′-CTTCTTGACTTCGTCG-3′) and oligodT (5′-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT-3′) primers. The amplification of the 5′ end was done by using the 5′ rapid amplification of cDNA ends System for Rapid Amplification of cDNA Ends (Invitrogen) following the manufacturer's protocol. A coding sequence of 1,191 bp was deduced from the three cloning steps, encompassing an ORF of 1,041 bp. By using oligonucleotides designed from 5′ and 3′ ends of the predicted sequence (5′-CTTGCCCTGTACGGGTACCGTC-3′ and 5′-GTCGCTTATGCCCTATTACCTATCAACC-3′), an amplicon of the expected length and sequence was obtained from total cDNA from pheromone glands, demonstrating the expression of the predicted mRNA in T. pityocampa.
Functional Assay.
Gene-specific primers (5′-GACTAAGATCTATGGCGCCGAACA CACGAGAGAAC-3′ and 5′-CATGGCGGCCGCGCCCTATTACCTATCAACCGTCG-3′), encompassing BglII and NotI restriction sites, respectively, were designed to amplify the ORF of Tpi-PGFAD using OneStep RT-PCR (Qiagen). The PCR product was digested, purified by gel electrophoresis, and ligated to pYEXTHS-BN (25) between the BamHI and NotI sites. The plasmid was transformed into DH5α competent cells, amplified, purified, and analyzed by sequencing.
An elo1Δ/ole1Δ (MATa elo1::HIS3 ole1::LEU2 ade2 his3 leu2 ura3) yeast strain was cultured as described (26) and transformed with the isolated ORF of T. pityocampa inserted in the pYEXTHS vector by standard methods (42) to produce the YMS1 yeast strain. Transformed cells were grown on selective medium plates with 2% glucose, 0.5 mM unsaturated fatty acids (oleate and palmitoleate), and 0.2 mM adenine sulfate, at 30°C for 48 h. A single colony from the synthetic dextrose (SD) medium plate was then inoculated into 2 ml of sterile yeast extract-peptone-dextrose medium with adenine containing 0.5 mM of CuSO4 and grown at 30°C for 48 h with shaking at 300 rpm, to confirm the cell viability without exogenous monounsaturated fatty acids. In the specified experiments, 0.5 mM 11-hexadecynoic acid was also present in the liquid medium. Cells were centrifuged, washed, and extracted with chloroform/methanol (2:1), and the lipid extracts were methanolyzed. Derivatization with dimethyl disulfide was carried out as reported (27). The extracts were analyzed, at −70 eV, on a Fisons gas chromatograph (8000 series) coupled to a Fisons MD-800 mass selective detector. The system was equipped with a nonpolar Hewlett Packard HP-1 capillary column (30 m × 0.25 mm i.d. × 0.25 μm stationary phase) by using the following temperature programs: from 80°C to 300°C at 2°C/min (fatty acid methyl ester analyses); from 80°C to 200°C at 5°C/min and then to 310°C at 7°C/min and held at 310°C for 15 min (dimethyl disulfide adduct analyses).
Protein Expression.
Preparation of protein extracts, SDS/PAGE, and Western blotting were carried out as reported (19).
Acknowledgments
We thank ICONA for collecting the insects and Dr. X. Matabosch for his help with tissue dissections. This work was funded by Spanish Ministry of Science and Technology Grant AGL2001-0585, a predoctoral fellowship (to M.S.), Spanish Ministry of Science and Education Grant BIO2005-00840, and Generalitat de Catalunya Grant 2005SGR-01063.
Abbreviations
- GC/MS
gas chromatography coupled to mass spectrometry
- Tpi-PGFAD
T. pityocampa pheromone gland fatty acid desaturase gene.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EF150363).
References
- 1.Tocher DR, Leaver MJ, Hodgson PA. Prog Lipid Res. 1998;37:73–117. doi: 10.1016/s0163-7827(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 2.Hayward SA, Murray PA, Gracey AY, Cossins AR. Adv Exp Med Biol. 2007;594:132–142. doi: 10.1007/978-0-387-39975-1_12. [DOI] [PubMed] [Google Scholar]
- 3.Hao G, Liu W, O'Connor M, Roelofs W. Insect Biochem Mol Biol. 2002;32:961–966. doi: 10.1016/s0965-1748(01)00176-x. [DOI] [PubMed] [Google Scholar]
- 4.Hao G, O'Connor M, Liu W, Roelofs WL. J Insect Sci. 2002;2:1–7. doi: 10.1093/jis/2.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knipple D, Rosenfield C, Miller SJ, Liu W, Tang J, Ma PWK, Roelofs WL. Proc Natl Acad Sci USA. 1998;95:15287–15292. doi: 10.1073/pnas.95.26.15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Jiao H, Murray NC, O'Connor M, Roelofs WL. Proc Natl Acad Sci USA. 2002;99:620–624. doi: 10.1073/pnas.221601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Jiao H, O'Connor M, Roelofs WL. Insect Biochem Mol Biol. 2002;32:1489–1495. doi: 10.1016/s0965-1748(02)00069-3. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Rooney AP, Xue B, Roelofs WL. Gene. 2004;342:303–311. doi: 10.1016/j.gene.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Moto K, Suzuki MG, Hull JJ, Kurata R, Takahashi S, Yamamoto M, Okano K, Imai K, Ando T, Matsumoto S. Proc Natl Acad Sci USA. 2004;101:8631–8636. doi: 10.1073/pnas.0402056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez S, Hao G, Liu W, Piña B, Rooney AP, Camps F, Roelofs WL, Fabriàs G. Insect Biochem Mol Biol. 2004;34:1315–1328. doi: 10.1016/j.ibmb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero A, Camps F, Coll J, Riba M, Einhorn J, Descoins C, Lallemand JY. Tetrahedron Lett. 1981;22:2013–2016. [Google Scholar]
- 12.Quero C, Malo EA, Fabrias G, Camps F, Lucas P, Renou M, Guerrero A. J Chem Ecol. 1997;23:713–726. [Google Scholar]
- 13.Fabriàs G, Arsequell G, Camps F. Insect Biochem. 1989;19:177–181. doi: 10.1002/arch.940140106. [DOI] [PubMed] [Google Scholar]
- 14.Arsequell G, Fabriàs G, Camps F. Arch Insect Biochem Physiol. 1990;14:47–56. doi: 10.1002/arch.940140106. [DOI] [PubMed] [Google Scholar]
- 15.Fabriàs G, Barrot M, Camps F. Insect Biochem Mol Biol. 1995;25:655–660. [Google Scholar]
- 16.Barrot M, Fabriàs G, Camps F. Tetrahedron. 1994;50:9789–9796. [Google Scholar]
- 17.Villorbina G, Rodríguez S, Camps F, Fabriàs G. Insect Biochem Mol Biol. 2003;33:155–161. doi: 10.1016/s0965-1748(02)00186-8. [DOI] [PubMed] [Google Scholar]
- 18.Roelofs WL, Liu W, Hao G, Jiao H, Rooney AP, Linn CE., Jr Proc Natl Acad Sci USA. 2002;99:13621–13626. doi: 10.1073/pnas.152445399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serra M, Piña B, Bujons J, Camps F, Fabriàs G. Insect Biochem Mol Biol. 2006;36:634–641. doi: 10.1016/j.ibmb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Sperling P, Lee M, Girke T, Zahringer U, Stymne S, Heinz E. Eur J Biochem. 2000;267:3801–3811. doi: 10.1046/j.1432-1327.2000.01418.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Lenman M, Banas A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson PO, et al. Science. 1998;280:915–918. doi: 10.1126/science.280.5365.915. [DOI] [PubMed] [Google Scholar]
- 22.Knipple DC, Rosenfield CL, Nielsen R, You KM, Jeong SE. Genetics. 2002;162:1737–1752. doi: 10.1093/genetics/162.4.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Ma PW, Marsella-Herrick P, Rosenfield CL, Knipple DC, Roelofs W. Insect Biochem Mol Biol. 1999;29:435–443. doi: 10.1016/s0965-1748(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfield CL, You KM, Marsella-Herrick P, Roelofs WL, Knipple DC. Insect Biochem Mol Biol. 2001;31:949–964. doi: 10.1016/s0965-1748(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 25.Holz C, Hesse O, Bolotina N, Stahl U, Lang C. Protein Expr Purif. 2002;25:372–378. doi: 10.1016/s1046-5928(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 26.Schneiter R, Tatzer V, Gogg G, Leitner E, Kohlwein SD. J Bacteriol. 2000;182:3655–3660. doi: 10.1128/jb.182.13.3655-3660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buser HR, Arn H, Guerin P, Rauscher S. Anal Chem. 1983;55:818–822. [Google Scholar]
- 28.Reed DW, Polichuk DR, Buist PH, Ambrose SJ, Sasata RJ, Savile CK, Ross ARS, Covello PS. J Am Chem Soc. 2003;125:10635–10640. doi: 10.1021/ja036489o. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson AS, Thomaeus S, Hamberg M, Stymne S. Eur J Biochem. 2004;271:2991–2997. doi: 10.1111/j.1432-1033.2004.04231.x. [DOI] [PubMed] [Google Scholar]
- 30.Cahoon EB, Schnurr JA, Huffman EA, Minto RE. Plant J. 2003;34:671–683. doi: 10.1046/j.1365-313x.2003.01757.x. [DOI] [PubMed] [Google Scholar]
- 31.Li XC, Jacob MR, ElSohly HN, Nagle DG, Smillie TJ, Walker LA, Clark AM. J Nat Prod. 2003;66:1132–1135. doi: 10.1021/np030196r. [DOI] [PubMed] [Google Scholar]
- 32.Electr S, Keith AD. Proc Natl Acad Sci USA. 1972;69:1353–1357. doi: 10.1073/pnas.69.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Wang G, Lin H, Huang CH. J Biol Chem. 1998;273:19009–19018. doi: 10.1074/jbc.273.30.19009. [DOI] [PubMed] [Google Scholar]
- 34.Wisnieski BJ, Kiyimoto RK. J Bacteriol. 1972;109:186–195. doi: 10.1128/jb.109.1.186-195.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abad JL, Rodriguez S, Camps F, Fabrias G. J Org Chem. 2006;71:7558–7564. doi: 10.1021/jo060789h. [DOI] [PubMed] [Google Scholar]
- 36.Abad JL, Villorbina G, Fabriàs G, Camps F. J Org Chem. 2004;69:7108–7113. doi: 10.1021/jo049320h. [DOI] [PubMed] [Google Scholar]
- 37.Abad JL, Serra M, Camps F, Fabrias G. J Org Chem. 2007;72:760–764. doi: 10.1021/jo061592s. [DOI] [PubMed] [Google Scholar]
- 38.Shanklin J, Cahoon EB. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- 39.Jurenka RA, Subchev M, Abad JL, Choi MY, Fabrias G. Proc Natl Acad Sci USA. 2003;100:809–814. doi: 10.1073/pnas.0236060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurenka RA. Cell Mol Life Sci. 1997;53:501–505. doi: 10.1007/s000180050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roelofs WL, Rooney AP. Proc Natl Acad Sci USA. 2003;100:9179–9184. doi: 10.1073/pnas.1233767100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito H, Fukuda Y, Murata K, Kimura A. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar S, Tamura K, Jakobsen IB, Nei M. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- 44.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]