Abstract
For many years, β-adrenergic receptor antagonists (β-blockers or βAR antagonists) have provided significant morbidity and mortality benefits in patients who have sustained acute myocardial infarction. More recently, β-adrenergic receptor antagonists have been found to provide survival benefits in patients suffering from heart failure, although the efficacy of different β-blockers varies widely in this condition. One drug, carvedilol, a nonsubtype-selective βAR antagonist, has proven particularly effective in the treatment of heart failure, although the mechanism(s) responsible for this are controversial. Here, we report that among 16 clinically relevant βAR antagonists, carvedilol displays a unique profile of in vitro signaling characteristics. We observed that in β2 adrenergic receptor (β2AR)-expressing HEK-293 cells, carvedilol has inverse efficacy for stimulating Gs-dependent adenylyl cyclase but, nonetheless, stimulates (i) phosphorylation of the receptor's cytoplasmic tail on previously documented G protein-coupled receptor kinase sites; (ii) recruitment of β-arrestin to the β2AR; (iii) receptor internalization; and (iv) activation of extracellular regulated kinase 1/2 (ERK 1/2), which is maintained in the G protein-uncoupled mutant β2ART68F,Y132G,Y219A (β2ARTYY) and abolished by β-arrestin2 siRNA. Taken together, these data indicate that carvedilol is able to stabilize a receptor conformation which, although uncoupled from Gs, is nonetheless able to stimulate β-arrestin-mediated signaling. We hypothesize that such signaling may contribute to the special efficacy of carvedilol in the treatment of heart failure and may serve as a prototype for a new generation of therapeutic β2AR ligands.
Keywords: β-adrenergic receptor, antagonist, ERK 1/2, scaffold, internalization
Seven transmembrane receptors (7TMRs), are one of the most important target classes of therapeutic agents, accounting for >30% of all prescription medications (1). Traditionally, ligands for such receptors have been classified as agonists, which promote signaling through activation of heterotrimeric G proteins and generation of second messengers such as cyclic adenosine monophosphate (cAMP), or antagonists that block such stimulation (2). Recent work, however, has suggested that this simple classification is inadequate and that receptors may exist in multiple active conformations, each of which could display a distinct signaling profile (3–6).
Moreover, it has recently been appreciated that, in addition to signaling through G proteins, 7TMRs can also use the multifunctional adaptor proteins β-arrestins 1 and 2 to activate cellular pathways (7, 8). These proteins were originally discovered to bind to activated GRK-phosphorylated receptors and thus block or “desensitize” further receptor-stimulated G protein activation (9). Consequently, β-arrestins exist as bifunctional cellular mediators: even as they terminate G protein signaling, they can function as scaffolds for signaling networks such as mitogen-activated protein kinases (MAPK) including ERK 1/2 (10, 11), cJun N-terminal kinase (JNK3) (12), and p38 kinase (13) as well as phosphatidylinositol 3 kinase (PI3K) and Akt (14–16). Recent work also suggests that β-arrestin may have significant antiapoptotic roles within the cell (10, 14, 17), although the exact mechanisms behind this regulation are largely unknown.
Ligands targeting 7TMRs such as βAR antagonists are used therapeutically in numerous conditions. By virtue of their ability to block the deleterious G protein-mediated effects of excess catecholamine stimulation in the heart and other organs, βAR antagonists have become important therapeutic tools for a variety of cardiovascular conditions including hypertension (18), angina (19), post-acute myocardial infarction (AMI) (20) and heart failure (21). The βAR antagonist carvedilol recently was demonstrated to significantly reduce morbidity and mortality in heart failure and in post-AMI patients (22–25). Moreover, although controversial, some evidence suggests carvedilol may possess unique survival advantages in heart failure over other βAR antagonists (23). The mechanisms of such advantages are currently unknown but have been variously ascribed to ancillary properties of carvedilol including antioxidant (26), antiinflammatory (27), antiproliferative (28), and antiarrhythmic abilities (29) as well as its capacity to serve as an α1 adrenergic receptor antagonist (30).
Given recent advances in understanding 7TMR signaling and regulation, we examined a subset of 16 known βAR antagonists for both AC and ERK 1/2 efficacy at the β2AR in simple cellular systems. This subset encompassed a wide range of clinically used agents including both nonselective and subtype-selective ligands. The results suggest a unique profile of activities for carvedilol that might explain, in part, its positive therapeutic attributes and promote development of β2AR ligands that antagonize Gs-mediated signaling while stimulating β-arrestin-mediated signaling.
Results
cAMP Accumulation.
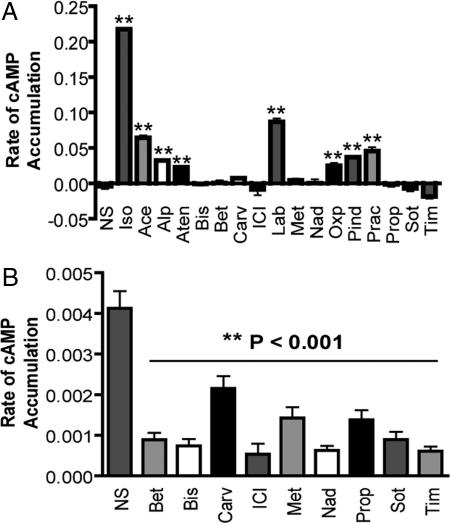
Typically, receptor ligands have been classified as either agonist (full or partial) or antagonist with respect to G protein-coupling efficiency. Work over the last 20 years has expanded this classification to include the concept of inverse agonism, and it has been observed that numerous classical neutral antagonists actually act as either partial agonists or inverse agonists (2). Here, we used the live-cell biosensor ICUE2 to assess Gs-dependent cAMP efficacy at the β2AR (31). Acebutolol, alprenolol, atenolol, labetalol, oxprenolol, pindolol, and practolol displayed weak partial agonism for Gs-dependent AC activation (Fig. 1A). Ligands that did not stimulate significant cAMP generation were further analyzed for inverse agonism. Each of these (betaxolol, bisoprolol, carvedilol, ICI 118,551, metoprolol, nadolol, propranolol, sotalol, and timolol) decreased constitutive cAMP accumulation and are thus inverse agonists (Fig. 1B). As described (2), we found no neutral antagonists, indicating that this classification may be an artifact of low assay sensitivity and that most if not all βAR ligands have some level of efficacy.
Fig. 1.
cAMP responses monitored by ICUE2. HEK-293 cells stably expressing β2AR and the cAMP biosensor ICUE2 were treated for 2 min with a panel of ligands described as β2AR antagonists. (A) cAMP agonism was measured as the rate of change of the ICUE2 FRET ratio corresponding to the rate of cAMP accumulation. Ligands that did not induce cAMP generation were tested for inverse agonism in the same cells. (B) These cells exhibit constitutive β2AR activity that, although too weak to generate high basal cAMP, causes a rapid increase in cAMP when phosphodiesterases are inhibited with isoxybutylmethylxanthine (IBMX). This effect is receptor-specific, because there is no IBMX-induced cAMP increase in cells lacking overexpressed receptor (data not shown). We measured inverse agonism by pretreating cells with ligand for 5 min and quantifying the rate of cAMP increase for 30 sec after IBMX treatment. Inverse agonists are those ligands that do not stimulate cAMP accumulation on their own and decrease the rate of IBMX-induced cAMP accumulation caused by constitutive receptor activity. Data represent mean ± SE from five independent experiments. **, P < 0.001 vs. nonstimulated cells (NS).
ERK 1/2 Activation.
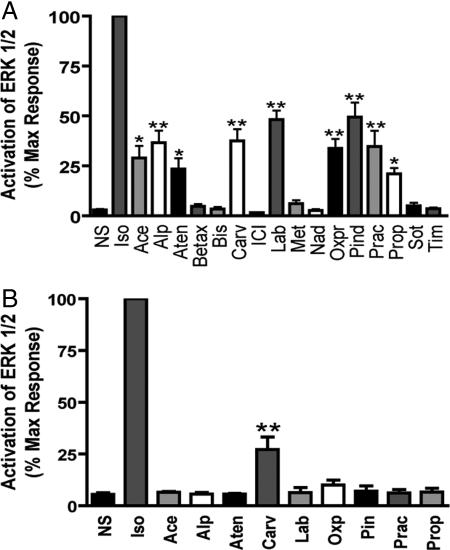
Recent work has demonstrated that β-arrestins can serve as scaffolds that activate signaling networks including ERK 1/2 (10, 11) independent of G protein activity (25). Although none of the βAR antagonists we tested led to ERK1/2 activation in untransfected HEK-293 cells, we observed that in HEK-293 cells stably expressing 2 pmol/mg β2AR, a wide range of ERK 1/2 activation responses can be elicited by different βAR antagonists (Fig. 2A). Acebutolol, atenolol, alprenolol, carvedilol, labetalol, oxprenolol, pindolol, practolol, and propranolol all activate ERK to varying degrees. To define the role of G protein stimulation in these ERK1/2 responses, we used a mutant β2AR that does not couple to G proteins but maintains the ability to stimulate MAP kinases: β2ARTYY (25). In cells stably expressing the mutant β2ARTYY, only carvedilol stimulated a significant ERK1/2 response (Fig. 2B). Through either receptor, carvedilol-stimulated pERK was insensitive to pertussis toxin pretreatment, excluding a role for Gi coupling in carvedilol-stimulated pERK [supporting information (SI) Fig. 6]. Furthermore, carvedilol-stimulated pERK was completely blocked by pretreatment with the β2AR selective antagonist ICI 118,551 (data not shown), demonstrating β2AR specificity.
Fig. 2.
ERK activation in β2AR and β2ARTYY stable cells. HEK-293 cells stably expressing β2AR (A) or β2ARTYY (B) were stimulated with the panel of β2AR ligands used in Fig. 1 at 10 μM for 5 min, and cell lysates were analyzed for pERK and ERK by Western blot. pERK was normalized to total ERK protein. Data represent mean ± SE of at least three independent experiments done in duplicate. Quantification of pERK bands is as a percentage of maximal activity observed for isoproterenol. *, P < 0.05 vs. NS, **, P < 0.001 vs. NS.
β2AR Phosphorylation.
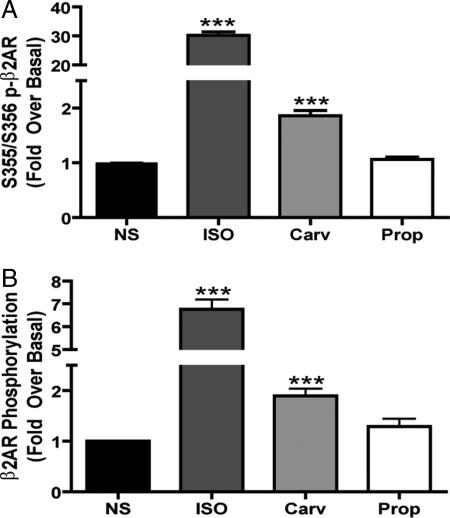
We then focused our screen on carvedilol and propranolol, which were the only βAR antagonists that functioned as inverse agonists for Gs-dependent AC activation but stimulated ERK 1/2 activation. Previous work demonstrated that the β2AR cytoplasmic tail must be phosphorylated by GRKs in order for β-arrestin to be recruited to the receptor (32). Using both a phospho-specific antibody identifying GRK phosphorylation sites and 32P metabolic labeling, we observed that carvedilol but not propranolol can stimulate significant β2AR phosphorylation. A 30-min treatment with carvedilol stimulated a 1.9 ± 0.09-fold increase in receptor phosphorylation at the known GRK sites, serine 355/356 (Fig. 3A) and a 1.9 ± 0.1-fold increase in global receptor phosphorylation (Fig. 3B). Carvedilol-mediated receptor phosphorylation was blocked by propranolol pretreatment (data not shown).
Fig. 3.
β2AR phosphorylation stimulated by carvedilol. HEK-293 cells stably expressing β2AR were stimulated with 10 μM of ligand for 30 min, and cell lysates were either analyzed for receptor phosphorylation by Western blot (A) or immunoprecipitated with anti-FLAG beads and analyzed by 32P metabolic labeling (B). Data represent mean ± SE of at least five independent experiments. ***, P < 0.0001 vs. NS.
β-Arrestin Recruitment.
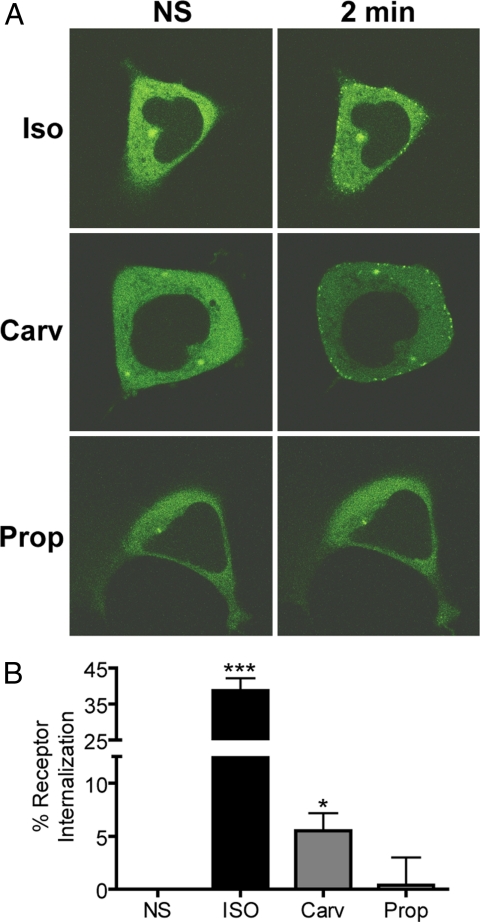
The β2AR exhibits a transient, low-affinity interaction with β-arrestins and undergoes rapid recycling to the plasma membrane after internalization, a pattern known as “Class A” recruitment. “Class B” receptors such as the vasopressin V2 receptor (V2R) have a more prolonged interaction with β-arrestin and recycle to the plasma membrane slower than Class A receptors (33). One method for increasing β-arrestin affinity, and therefore assay sensitivity, is to construct chimeric Class A receptors that possess a Class B receptor cytoplasmic tail, such as the β2AR-V2R receptor chimera (34). As visualized by confocal microscopy, both isoproterenol and carvedilol stimulated recruitment of β-arrestin2-GFP to the β2AR-V2R receptor chimera, whereas propranolol did not (Fig. 4A). Alprenolol, labetalol, and ICI 118,551 were also unable to stimulate β-arrestin2–GFP recruitment (data not shown). Furthermore, carvedilol-stimulated β-arrestin recruitment was blocked by pretreatment with either ICI 118,551 or propranolol (data not shown), demonstrating β2AR specificity.
Fig. 4.
β-arrestin2-GFP translocation to the β2AR-V2R and receptor internalization stimulated by carvedilol. HEK-293 cells transiently expressing the β2AR-V2R chimera were stimulated for 2 min with either isoproterenol (Iso), carvedilol (Carv), or propranolol (Prop). (A) β-arrestin2-GFP translocation to the β2AR-V2R was then monitored by confocal microscopy. Images are representative of six independent experiments. (B) HEK-293 cells stably expressing β2AR were stimulated with 10 μM of ligand for 30 min and assayed for internalization by fluorescence-activated cell sorting. Data represent mean ± SE of five independent experiments done in duplicate. *, P < 0.05 vs. NS, ***, P < 0.0001 vs. NS.
β2AR Internalization.
Previous studies have demonstrated that β-arrestins can serve as adaptors for AP-2 and clathrin (35–37), which bring activated receptors to clathrin-coated pits for endocytosis and facilitate receptor internalization. Carvedilol, but not propranolol, induces receptor internalization as assessed by fluorescence-activated cell sorting. Carvedilol stimulated 5.5% ± 1.7 receptor internalization, whereas the full agonist isoproterenol stimulated 38.7% ± 3.5 receptor internalization (Fig. 4B).
β-Arrestin2-Mediated ERK Activation.
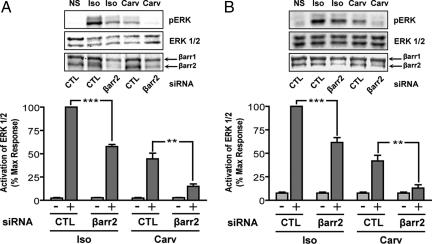
To test the potential role of β-arrestin in carvedilol-stimulated cell signaling, we analyzed ERK 1/2 activation after depletion of β-arrestin2 by siRNA in HEK-293 cells stably expressing β2AR. For isoproterenol, pERK was reduced by 42.3 ± 2.2% at 5 min, whereas for carvedilol, pERK was reduced by 71.0 ± 4.5% (Fig. 5A). In HEK-293 cells stably expressing β2ARTYY, pERK for isoproterenol was reduced by 38.5 ± 5.2% at 5 min, whereas for carvedilol, pERK was reduced by 70.1 ± 4.4% after β-arrestin2 silencing (Fig. 5B). Both of these results correlated with the overall efficiency of the β-arrestin2 siRNA to lower cellular levels of β-arrestin2 by ≈70% (data not shown). A second siRNA sequence targeting β-arrestin2 gave similar results, demonstrating specificity of the silencing effect (data not shown). Of note, isoproterenol-stimulated ERK activation seen in cells expressing β2ARTYY after siRNA silencing of β-arrestin2 was due to G protein-stimulated ERK activation from endogenous β2ARs in HEK-293 and is not mediated by the mutant receptors (25).
Fig. 5.
Carvedilol-stimulated ERK 1/2 phosphorylation is abolished by siRNA targeting β-arrestin2. HEK-293 cells stably expressing β2AR (A) or β2ARTYY (B) were stimulated with 10 μM isoproterenol (Iso) or carvedilol (Carv) in the presence of either control siRNA (CTL) or siRNA targeting β-arrestin2 (βarr2) for 5 min, and cell lysates were analyzed for pERK, ERK, and β-arrestin 1/2 by Western blot. Data represent mean ± SE of four independent experiments done in duplicate. Quantification of pERK bands is as a percentage of maximal activity observed for isoproterenol. **, P < 0.01; *** P < 0.001.
Discussion
Here, we report that among 16 βAR antagonists, a diverse spectrum of efficacies was observed for both Gs-dependent and β-arrestin-dependent cellular signaling (SI Table 1). Additionally, we identify one compound, carvedilol, that possesses the unique signaling profile of negative efficacy for Gs-dependent AC activation but positive efficacy for β-arrestin-dependent ERK 1/2 activation. Moreover, carvedilol stimulates phosphorylation of the β2AR, β-arrestin translocation to the receptor, and receptor internalization, all of which are characteristic of β-arrestin-mediated cellular processes. Thus, carvedilol acts as a “biased” ligand (38) signaling via β-arrestin-dependent ERK 1/2 activation in the absence of G protein activation. We hypothesize that this bias may help explain carvedilol's unique clinical effectiveness in heart failure and other cardiovascular diseases.
The concept of ligand bias challenges the traditional paradigm of 7TMR ligand characterization based on “intrinsic efficacy,” the amount of stimulus per receptor molecule elicited by a ligand upon binding (39). Implicit in this classification is the assumption that a given ligand should be equally effective at stimulating all cellular responses for a given receptor (39, 40). However, much work over the last 15 years has demonstrated that a single ligand can have differential intrinsic efficacies for various effector systems downstream of a given receptor (3, 39, 41–43). Consequently, a given ligand's pharmacologic efficacy, or its ability to produce a desired therapeutic effect, may not be fully explained by its ability to stimulate a single receptor-mediated signaling pathway. The recognition that pharmacologic efficacy cannot be encompassed by the simple agonist/antagonist classification has not, to date, been correlated with insight into associated clinical outcomes of biased ligands.
Recently, it has been appreciated that many receptors including the β2AR can exist in multiple “active” conformations after ligand binding (3–6). These variable conformations may lead to widely differing cellular outcomes and may help explain the diverse signaling profiles we observed with a variety of βAR antagonists. Viewed in the context of this report, it seems likely that carvedilol stabilizes distinct receptor conformations from other βAR antagonists. Thus, it is perhaps not surprising that these compounds range in their clinical efficacy as well (21). We postulate that the diverse signaling profiles observed in this study indicate that ligand bias may play a role in determining the effectiveness of pharmacologic agents targeting 7TMRs.
One of the major paradigm shifts in cardiovascular medicine was the innovation that βAR antagonists could be an effective therapy for the treatment of heart failure (44). This originated from the observation that activation of the sympathetic nervous system may be fundamental to the progression of heart failure (45). Consequently, it was hypothesized that pharmacologic blockade of the sympathetic nervous system, in particular of the β1AR and β2AR, could slow the progression of heart failure (46). It was recognized quite early that different βAR antagonists possessed differing clinical efficacies in heart failure, and their effectiveness could not simply be attributed to a class effect (47, 48). Currently, only three agents, carvedilol, metoprolol succinate, and bisoprolol are approved for the treatment of heart failure in the United States (48). Although their relative efficacies are unknown, some evidence suggests that carvedilol may possess survival advantages over other βAR antagonists (23).
Survival advantages observed with carvedilol treatment in cardiovascular diseases including heart failure (23) and post-AMI (20) have been ascribed to a multitude of ancillary properties of carvedilol as outlined earlier (26–30). However, controversy still exists over whether any of these properties can sufficiently explain carvedilol's clinical efficacy. We hypothesize that the unique signaling profile exhibited by carvedilol may, in part, explain its distinctive clinical efficacy. This hypothesis is supported by recent animal work demonstrating that β-arrestin-dependent signaling can be cardioprotective in the presence of chronic catecholamine stimulation, as is the case in heart failure, whereas G protein-dependent signaling may be cardiotoxic under these same conditions (49). These in vivo experiments revealed that, in these conditions, the loss of β-arrestin-mediated signaling through the β1 adrenergic receptor resulted in increased apoptosis and cardiac deterioration. This work provides strong evidence that a biased ligand such as carvedilol, which antagonizes G protein-mediated signaling while simultaneously stimulating β-arrestin-mediated signaling, may have increased therapeutic potential over conventional βAR antagonists.
In our studies, only carvedilol possessed this unique profile of inverse agonism for Gs-dependent AC activation while concurrently stimulating β-arrestin-dependent ERK 1/2 activation. One possible caveat, however, is that all experiments were carried out by using exogenously expressed receptors because of the difficulty in detecting responses with endogenous receptors in our assays. Propranolol, which also acted as an inverse agonist for Gs-dependent AC activation, was able to weakly activate ERK 1/2 as observed by others (41–43). In our system, however, propranolol was unable to activate several characteristic processes of β-arrestin-dependent signaling. These observations suggest the possibility of yet another signaling pathway to ERK 1/2 activation that is both G protein- and β-arrestin-independent and that can be stimulated by propranolol. The functional consequences of such a pathway are unknown but likely differ from both the G protein- and β-arrestin-dependent pathways.
In the current report, we monitored only ERK 1/2 activation as a readout for β-arrestin-dependent signaling. Because β-arrestin is known to signal via a range of other signaling pathways, β-arrestin-biased ligands likely also stimulate β-arrestin-dependent, G protein-independent signaling via JNK3, p38 kinase, PI3K, or Akt. Biased ligands also possibly stimulate multiple levels of specificity such as GRK subtype-specific receptor phosphorylation and/or stabilization of specific β-arrestin conformations coupled to distinct functional outcomes. Furthermore, compounds could also be biased in the opposite direction from carvedilol, activating G protein-mediated pathways while simultaneously antagonizing β-arrestin-dependent signaling pathways (50). The physiological and clinical profiles of such agents might be distinct from those currently available. Ultimately these biased ligands may possess even greater efficacy in the treatment of various diseases while reducing undesirable effects and would represent a model for the development of 7TMR-targeted therapeutics.
The recognition that intrinsic efficacy is not simply a function of G protein-coupling efficacy and can vary greatly depending on the observed effector system has changed the definition of pharmacologic efficacy. Here, we have identified a signaling profile unique to carvedilol that may correlate with, or be responsible for, its unique clinical efficacy. In our study, carvedilol stimulates only very weak activation of β-arrestin-dependent signaling processes. Consequently, we propose that carvedilol could serve as a prototype for the design of compounds that, although possessing no efficacy for G protein stimulation, could nevertheless stimulate β-arrestin-dependent signaling to a greater extent than carvedilol. Biased ligands of this type would represent a new generation of therapeutic agents that could be targeted for any number of receptors. Although the therapeutic potential of these biased ligands remains to be demonstrated, the data presented herein suggest a potential direction in the treatment of cardiovascular disease and, in particular, of heart failure.
Materials and Methods
Materials.
Acebutolol, alprenolol, atenolol, betaxolol, ICI 118,551, isoproterenol, labetalol, metoprolol, nadolol, pindolol, propranolol, sotalol, and timolol were obtained from Sigma (St. Louis, MO). Bisoprolol and practolol were obtained from Mutual Pharmaceutical (Philadelphia, PA) and Ayerst Laboratories (Madison, NJ), respectively. Racemic carvedilol was generously provided by Dr. Richard Bond (University of Houston, Houston, TX).
Plasmids.
FLAG-β2AR/pcDNA3 (25) and β-arrestin2-GFP (25) were generated in our laboratory. The β2AR-V2R chimera receptor was a generous gift from Dr. Marc Caron (Duke University) (34).
Cell Culture.
HEK-293 lines stably expressing 2 pmol/mg β2AR or β2ARTYY were generated in our laboratory (25) and were maintained in MEM supplemented with 10% FBS, 1% penicillin/streptavidin, and 400 μg/ml G418 (Sigma).
ICUE2 cAMP Assay.
HEK-293 cells stably overexpressing both the human β2AR (25) and the cAMP biosensor ICUE2 (31) were generated and validated as described (31). Intracellular cAMP concentrations were measured as a FRET ratio: the CFP intensity (438/24 excitation and 483/32 emission bandpass filters; Semrock, Rochester, NY) over FRET intensity (542/27 emission filter). Experiments were performed on a NOVOstar plate reader (BMG, Durham, NC). Data shown are the change in FRET ratio before and after addition of ligand or, for the inverse agonism experiments, addition of isobutylmethylxanthine (IBMX). IBMX was used at 250 μM to inhibit PDE activity and allow detection of constitutive β2AR activity.
Immunoblotting.
Phospho-ERK and phospho-β2AR immunoblotting were carried out as described (25). pERK antibody was anti-phospho-p44/42 MAPK (1:3,000; Cell Signaling Technology, Beverly, MA) for Western blot. Total ERK1/2 was detected with anti-MAPK 1/2 (1:6000; Upstate Biotechnology, Lake Placid, NY) for Western blot. Antibodies recognizing the β2AR and phospho-β2AR (on S355/6) were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Rabbit polyclonal β-arrestin antibody (A1CT) was generated in our laboratory (51).
Metabolic Labeling and β-Arrestin Translocation Assays.
Metabolic labeling and β-arrestin translocation assays were accomplished according to previously described protocols (25). For β-arrestin translocation, HEK-293 cells were transiently transfected with the β2V2R chimera (34) and β-arrestin-2-GFP by using FuGENE (Roche, Indianapolis, IN). Images were taken at 1-min intervals for 10 min after stimulation.
Internalization and siRNA Silencing of Gene Expression.
Internalization and siRNA gene silencing were carried out with previously described siRNAs and methods (25), except for the addition of a second siRNA targeting β-arrestin2, which was 5′-CCAACCUCAUUGAAUUUGA-3′. To determine β-arrestin 2 protein silencing, endogenous β-arrestin 1/2 were detected with A1CT (1:3000) antibody, as described (52). Only experiments with validated silencing were analyzed; average silencing of β-arrestin2 was 69.9 ± 1.6%.
Statistical Analysis.
Statistical analyses performed were one-way ANOVA with post hoc Bonferroni tests, or Student's paired t tests. A P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Donna Addison and Elizabeth Hall for excellent secretarial assistance, Dr. Richard Bond for providing carvedilol, and Dr. Marc Caron for providing β2AR-V2R plasmid. This work was supported in part by National Institutes of Health Grants HL16037 and HL70631. J.W.W. was supported by a fellowship from the Sarnoff Cardiovascular Research Foundation, J.D.V. was supported by a fellowship from the American Heart Association, and R.J.L. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations
- βAR
β-adrenergic receptor
- 7TMR
7 transmembrane receptor
- GRK
G protein-coupled receptor kinase
- β2ARTYY
β2ART68F,Y132G,Y219A
- AC
adenylyl cyclase
- ERK 1/2
extracellular-regulated kinase 1/2
- NS
nonstimulated.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707936104/DC1.
References
- 1.Hopkins AL, Groom CR. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Kenakin T. Trends Pharmacol Sci. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Kenakin T. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 4.Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Proc Natl Acad Sci USA. 2001;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. J Biol Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- 6.Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, Zare RN, Kobilka B. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 7.Lefkowitz RJ, Shenoy SK. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 8.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 9.Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Cheng Z, Ma L, Pei G. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 14.Povsic TJ, Kohout TA, Lefkowitz RJ. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 15.Goel R, Phillips-Mason PJ, Raben DM, Baldassare JJ. J Biol Chem. 2002;277:18640–18648. doi: 10.1074/jbc.M108995200. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Revankar CM, Vines CM, Cimino DF, Prossnitz ER. J Biol Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 18.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Thom SM. Br Med J. 2004;328:634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, et al. Circulation. 2003;107:149–158. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 20.Kopecky SL. Am J Cardiol. 2006;98:1115–1119. doi: 10.1016/j.amjcard.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 21.Bristow MR. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 22.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 23.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, et al. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 24.Dargie HJ. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 25.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 26.Yue TL, Cheng HY, Lysko PG, McKenna PJ, Feuerstein R, Gu JL, Lysko KA, Davis LL, Feuerstein G. J Pharmacol Exp Ther. 1992;263:92–98. [PubMed] [Google Scholar]
- 27.Packer M. Prog Cardiovasc Dis. 1998;41:39–52. doi: 10.1016/s0033-0620(98)80030-3. [DOI] [PubMed] [Google Scholar]
- 28.Ohlstein EH, Douglas SA, Sung CP, Yue TL, Louden C, Arleth A, Poste G, Ruffolo RR, Jr, Feuerstein GZ. Proc Natl Acad Sci USA. 1993;90:6189–6193. doi: 10.1073/pnas.90.13.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naccarelli GV, Lukas MA. Clin Cardiol. 2005;28:165–173. doi: 10.1002/clc.4960280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bristow MR, Larrabee P, Minobe W, Roden R, Skerl L, Klein J, Handwerger D, Port JD, Muller-Beckmann B. J Cardiovasc Pharmacol. 1992;19(Suppl 1):S68–S80. doi: 10.1097/00005344-199219001-00014. [DOI] [PubMed] [Google Scholar]
- 31.DiPilato LM, Cheng X, Zhang J. Proc Natl Acad Sci USA. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 33.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 34.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 35.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 36.Krupnick JG, Goodman OB, Jr, Keen JH, Benovic JL. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 37.Laporte SA, Miller WE, Kim KM, Caron MG. J Biol Chem. 2002;277:9247–9254. doi: 10.1074/jbc.M108490200. [DOI] [PubMed] [Google Scholar]
- 38.Violin JD, Lefkowitz RJ. Trends Pharmacol Sci. 2007 doi: 10.1016/j.tips.2007.06.006. in press. [DOI] [PubMed] [Google Scholar]
- 39.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 40.Benovic JL, Staniszewski C, Mayor F, Jr, Caron MG, Lefkowitz RJ. J Biol Chem. 1988;263:3893–3897. [PubMed] [Google Scholar]
- 41.Baker JG, Hall IP, Hill SJ. Mol Pharmacol. 2003;63:1312–1321. doi: 10.1124/mol.63.6.1312. [DOI] [PubMed] [Google Scholar]
- 42.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galandrin S, Bouvier M. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 44.Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Br Heart J. 1975;37:1022–1036. doi: 10.1136/hrt.37.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bristow MR. N Engl J Med. 1984;311:850–851. doi: 10.1056/NEJM198409273111310. [DOI] [PubMed] [Google Scholar]
- 46.Fowler MB, Bristow MR. Am J Cardiol. 1985;55:120D–124D. doi: 10.1016/0002-9149(85)91066-5. [DOI] [PubMed] [Google Scholar]
- 47.Cruickshank JM. Int J Cardiol. 2007;120:10–27. doi: 10.1016/j.ijcard.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 48.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, et al. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 49.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 52.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.