Abstract
Little is known about the tyrosine kinase c-Src's function in the systemic circulation, in particular its role in arterial responses to hormonal stimuli. In human aortic and venous endothelial cells, c-Src is indispensable for 17β-estradiol (E2)-stimulated phosphatidylinositol 3-kinase/Akt/endothelial NO synthase (eNOS) pathway activation, a possible mechanism in E2-mediated vascular protection. Here we show that c-Src supports basal and E2-stimulated NO production and is required for E2-induced vasorelaxation in murine aortas. Only membrane c-Src is structurally and functionally involved in E2-induced eNOS activation. Independent of c-Src kinase activity, c-Src is associated with an N-terminally truncated estrogen receptor α variant (ER46) and eNOS in the plasma membrane through its “open” (substrate-accessible) conformation. In the presence of E2, c-Src kinase is activated by membrane ER46 and in turn phosphorylates ER46 for subsequent ER46 and c-Src membrane recruitment, the assembly of an eNOS-centered membrane macrocomplex, and membrane-initiated eNOS activation. Overall, these results provide insights into a critical role for the tyrosine kinase c-Src in estrogen-stimulated arterial responses, and in membrane-initiated rapid signal transduction, for which obligate complex assembly and localization require the c-Src substrate-accessible structure.
Keywords: endothelium, eNOS
The most biologically potent, endogenous estrogen, 17β-estradiol (E2), is able to enhance vascular NO bioavailability by chronically increasing endothelial NO synthase (eNOS) expression and acutely stimulating eNOS activity through estrogen receptor (ER)-dependent signal transduction, most notably involving the phosphatidylinositol 3-kinase/Akt/eNOS pathway in endothelial cells (EC). This notion serves as one fundamental explanation for E2-induced vascular protection against ischemia/reperfusion injury, atherosclerosis progression, and neointima formation. Although membrane ERα and membrane ER46 (a de novo truncated ERα) are now appreciated to initiate estrogen-stimulated, c-Src-dependent eNOS activation after acquiring c-Src activity (1–3), little is known about the molecular details of c-Src involvement and the importance of c-Src in estrogen-induced vasorelaxation.
In mammals, Src family kinases have at least eight structurally distinct members and pivotally regulate cell migration, adhesion, proliferation, differentiation, and survival (4). Among eight Src kinase family members, only c-Src, Fyn, and Yes are ubiquitously expressed. Phosphorylation of c-Src Y416 with concomitant Y527 dephosphorylation is a consequence of c-Src activation and often leads to full kinase activity (4). c-Src family members are location-sensitive, membrane-associated, nonreceptor tyrosine kinases. c-Src kinase is reversibly coupled to the inner leaflet of the plasma membrane via a myristate moiety covalently attached to the G2 residue and a polybasic cluster embedded in the N terminus (5). All Src tyrosine kinases have a characteristic structure including single Src homology (SH) 3, SH2, and protein tyrosine kinase domains. Cytosolic c-Src is often self-restrained to an inactive conformation by both intramolecular and intermolecular interactions between its SH2 domain and phospho-Y527 and between its SH3 domain and SH2-kinase linker domains. Disrupting the inhibitory conformation by Y527 dephosphorylation or protein binding to the SH2 or SH3 domains imparts c-Src with an open structure permissive for Y416 autophosphorylation and resultant kinase activation (6, 7).
c-Src kinase can phosphorylate ERα at Y537 in resting cells, and, in turn, the c-Src SH2 domain can directly bind to ERα phospho-Y537 (8, 9). The “open” conformation, normally prompted via Y527 dephosphorylation by protein tyrosine phosphatase (PTP) α, PTPγ, PTP1B, or SHP-2 (6, 7), governs molecular recognition of c-Src substrates and its binding partners and is necessary for the redistribution of c-Src from endosomes to focal adhesions (10). In this article we present the molecular dissection of c-Src membrane–ER46 interactions, including interdependence for membrane targeting. We also display, by use of aortas obtained from c-Src gene-deleted animals, the critical role of c-Src in estrogen-induced vascular responses.
Results
c-Src Requirement in Basal and E2-Induced Vasorelaxation.
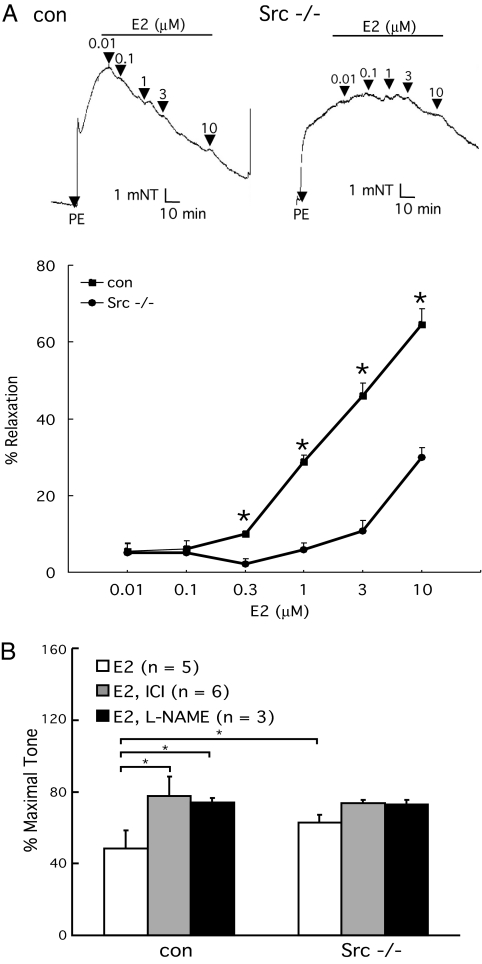
To determine whether c-Src plays a physiological role in E2-induced vasomotor responses, we analyzed the basal and E2-modulated vascular tone ex vivo by myography. E2 (10−6 to 3 × 10−5 M) induced 10–65% vasorelaxation of l-phenylephrine (PE)-preconstricted control aortic rings and 4–22% in c-Src−/− aortic rings (representative myographs shown in Fig. 1A). That is, E2-induced vasodilation was markedly blunted in aortic rings from c-Src gene-deleted mice. The observed relaxation was partially suppressed by Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME) (38 ± 2.6%, E2 = 3 × 10−5 M, n = 6, P < 0.05) and ICI 182,780 (66 ± 3.2%, E2 = 10−5 M, n = 5, P < 0.05), indicating that E2-enhanced NO bioavailability, through ER signaling, is at least partially responsible for the observed vasodilation (Fig. 1B). Endothelial denudation abolished E2-induced vasorelaxation in c-Src+/+ and c-Src−/− aortic rings, and sodium nitroprusside (NO donor, 10−6 M) produced comparable vasodilating responses in both [see supporting information (SI) Fig. 7]. These results suggest that the differential response to E2 (Fig. 1A) was due largely to the difference in endothelium-derived vasodilator production and that smooth muscle responses were identical. In intact, non-denuded rings, acetylcholine (10−6 M) responses were also similar (see SI Fig. 7). This indicates not only that the endothelium is intact and eNOS responses are preserved in c-Src−/− mice, but also that there is some specificity to the molecular complex that transduces estrogen-triggered signals. E2 stimulation of aortic rings was also performed in the presence of prostaglandin synthesis inhibition (indomethacin). Although the resting tone was greater in the presence of indomethacin, the vasodilating response to E2 was unaffected (data not shown). L-NAME pretreatment followed by PE challenge was also used to evaluate basal NO release. PE-induced, basal aortic tone in c-Src−/− rings was equal to that observed in L-NAME-treated wild-type rings (data not shown). These data implicate c-Src as a physiologically relevant modulator for basal and E2-induced vasorelaxation in murine aortas.
Fig. 1.
c-Src-dependent basal and E2-induced vasorelaxation. (A) Impaired E2 induced vasodilation in PE-preconstricted c-Src−/− aortas. Control and Src−/− thoracic aorta rings were isolated, preconstricted with 10−6 M PE, and treated with E2, and the vascular tone was recorded by vessel myograph system. (B) Partially NO- and receptor-dependent vasorelaxation (10−8 M E2, 10−5 M ICI 182,780, and 10−4 M L-NAME). *, P < 0.05 (two-tailed ANOVA).
E2-Induced c-Src Phosphorylation Status and c-Src Requirement in eNOS Activation.
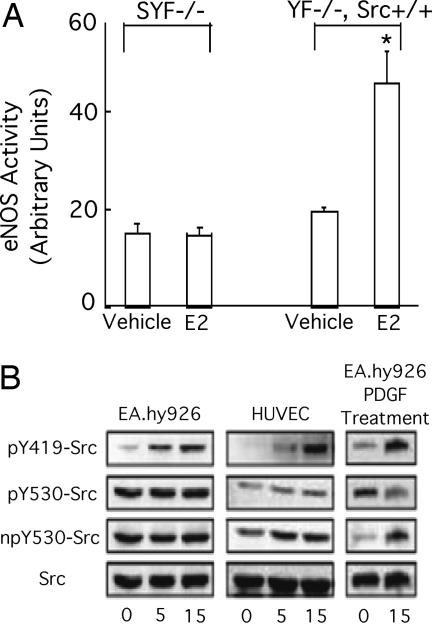
To document a c-Src requirement in E2-induced eNOS activation, we compared responsiveness in murine embryonic fibroblasts derived from embryos lacking all three major Src kinase members, Yes, Fyn, and c-Src (SYF−/−), to those observed in the same cells with c-Src reconstitution by stable c-Src expression (YF−/−). In triple knockout SYF−/− murine embryonic fibroblasts that heterologously express human ER46 and bovine eNOS, E2 failed to activate eNOS. However, E2 stimulation did increase eNOS activity (2.7 ± 0.4-fold) in YF−/−, Src+/+ cells (Fig. 2A). Expressed ER46 and eNOS levels in these transfected cells were equal (SI Fig. 8). Phosphorylation of c-Src Y419 (avian Y416) with concomitant Y530 (avian Y527) dephosphorylation is a consequence of c-Src activation and often leads to full kinase activity (4). In human umbilical vein ECs that contain both full-length ERα (referred to as ERα, unless otherwise indicated) and ER46, and EA.hy926 cells (an immortalized human umbilical vein EC line) that conditionally express ER46 only (2), E2 stimulated phosphorylation of c-Src on Y419 residue in 5–15 min while the levels of phospho-Y530 and non-phospho-Y530 remained unchanged (Fig. 2B). This is in contrast to PDGF-induced, parallel EC c-Src phosphorylation and dephosphorylation. Thus, the E2-induced eNOS activation requires the presence of c-Src, which becomes phosphorylated on Y419 in the absence of c-Src Y530 dephosphorylation.
Fig. 2.
Requirement of c-Src in ER46-mediated eNOS activation. (A) Activation of eNOS by E2 in reconstituted YF−/−, Src+/+ but not SYF−/− cells. Cells were transfected with eNOS and ER46, challenged with 30 nM E2 for 10 min, and subjected to eNOS activity assay. *, P < 0.05, n = 4. (B) Phosphorylation of Y419 without dephosphorylation of Y530 of c-Src by E2 in ECs. Pretreated cells were treated with 30 nM E2 for the indicated time, followed by lysate immunoblot with pY419-Src, pY530-Src, npY530 (non-phospho-Y530)-Src, and c-Src antibodies. PDGF (5 ng/ml) treatment demonstrates a typical, parallel Y419 phosphorylation and Y530 dephosphorylation response using the same antibodies.
ER46–c-Src Interdependence for Plasma Membrane Localization.
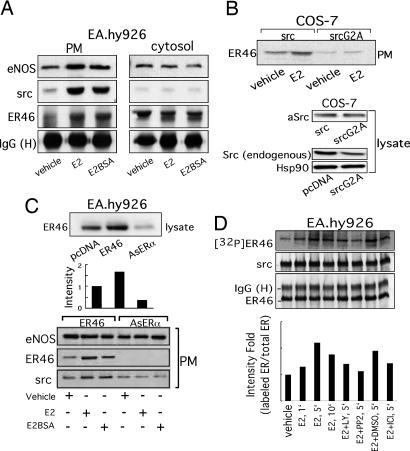
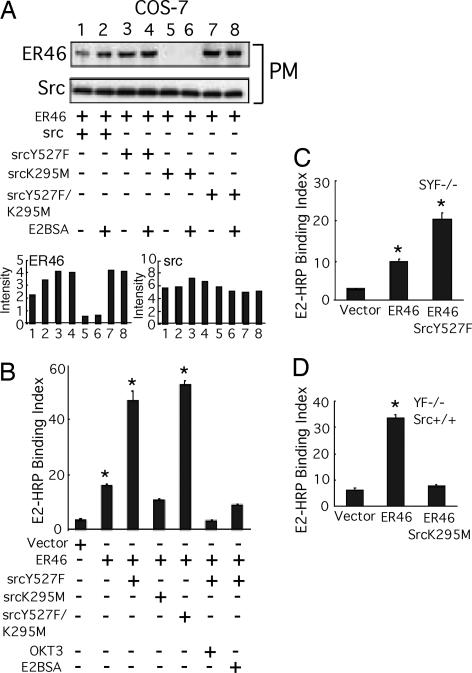
Several features of ER, c-Src, and eNOS membrane targeting have been described (3, 11, 12), but molecular interdependence of such has not been addressed. c-Src was coimmunoprecipitated with ER46 and eNOS from EA.hy926 plasma membranes but not from the cytosol. E2 or membrane-impermeant E2BSA enhanced the association of ER46, c-Src, and eNOS in the membrane (Fig. 3A). Decrease of ER46 expression (65%) with antisense ERα in EA.hy926 cells (Fig. 3C) or overexpression of myristoylation-deficient c-Src mutants in ER46-transfected COS-7 cells diminished the plasma membrane appearance of c-Src and ER46 (Fig. 3 B and C). The levels of eNOS in plasma membranes (Fig. 3C) and of total Hsp90 (Fig. 3B) in cell lysates were not altered by AsERα and SrcG2A expression, indicating the specificity of changes in targeted genes. Of note, expression of exogenous avian c-Src (aSrc) in COS-7 cells reduced the levels of endogenous c-Src. This may explain why heterologous c-Src expression apparently masked the role of endogenous c-Src in these studies (Figs. 3 and 4). These results indicate that, in addition to palmitoylation and myristoylation, known to be important for membrane localization of ER46 (2) and c-Src (5), respectively, ER46 and c-Src are interdependent for membrane incorporation, possibly because of their protein–protein interaction.
Fig. 3.
Assembly of a functional ER46–Src–eNOS macrocomplex in plasma membranes. (A) Complex formation in EC plasma membranes. Isolated plasma membranes and cytosol from E2-treated or E2BSA-treated (30 nM, 10 min) EA.hy926 cells were used for coimmunoprecipitation with ER antibody (F-10), followed by immunoblot with c-Src and eNOS antibodies. (B and C) Interdependent incorporation of c-Src and ER46 in plasma membranes. Electroporated EA.hy926 and Lipofectamine-transfected COS-7 cells were treated with 30 nM E2 or E2BSA for 10 min, fractionated, and immunoblotted. (D) Phosphorylation of ER46 by c-Src in vitro. ER46-centered complexes were immunoprecipitated with ER antibody (F-10) from EA.hy926 cell lysate and treated with 30 nM E2 and indicated inhibitors (10 μM LY 294002, 10 μM ICI 182,780, and 10 μM PP2) in the presence of [γ-32P]ATP in vitro, followed by protein fractionation, autoradiography, and immunoblot.
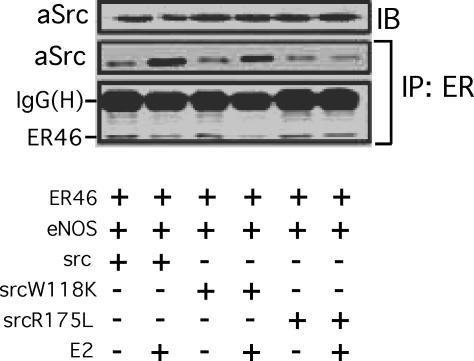
Fig. 4.
Coimmunoprecipitation of ER with c-Src variants. COS-7 cells were transfected with ER46, eNOS, and the indicated avian c-Srcs, E2-deprived for 48 h, serum-starved for 12 h, and stimulated with 33 nM E2 for 10 min. Cell lysates were used for immunoprecipitation with F-10 antibody, and the coimmunoprecipitated avian c-Src was examined by immunoblotting. SrcW118K, c-Src SH3 mutant; SrcR175L, c-Src SH2 mutant.
ER46–c-Src Interaction Domains.
To define ER46 and c-Src domains that confer the aforementioned, critical interactions, a series of avian (to distinguish between endogenous and transfected) c-Src mutants were cotransfected with ER46 (and, in activation experiments, with eNOS) into COS-7 cells, followed by coimmunoprecipitations. Before doing so, metabolic labeling experiments were performed to determine whether E2 induces a c-Src-dependent phosphorylation of ER46 in EC. In EA.hy926 cell ER46–Src–eNOS immunocomplexes (Fig. 3D), E2 stimulates ER46 phosphorylation in vitro, a process blocked by both the Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) (95%) and the pure ER antagonist ICI 182,780 (70%). Because Y537 is the only known c-Src substrate residue in ERα (9, 13), it is likely that this phosphorylation event enhances, or is even responsible for, the ER46–c-Src interaction in EC. This is supported by our finding that, after E2 stimulation, ER46 antibody coimmunoprecipitated wild-type aSrc and the SH3 mutant aSrcW118L, but not the SH2 mutant aSrcR175L (Fig. 4), one shown to abrogate c-Src interactions with phosphotyrosine-containing proteins (14). As noted above, c-Src is often self-restrained to an inactive conformation by both intramolecular and intermolecular interactions between its SH2 domain and phospho-Y530 and between its SH3 domain and SH2-kinase linker domains. To determine whether the c-Src substrate-accessible open structure and/or kinase activation are required for c-Src localization in membrane ER46 complexes, the avian c-Src constructs including wild-type Src, SrcY527F (open and active), SrcK295M (kinase-dead), and SrcY527F/K295M (substrate-accessible but kinase-dead) were separately expressed in ER46-reconstituted COS-7 cells (Fig. 5A). Wild-type Src expression supported E2BSA-stimulated membrane ER46 recruitment. Conversely, SrcK295M, a kinase-dead mutant that is likely to adapt an inactive conformation, radically abolished (95%) membrane localization of ER46 in the presence and absence of E2BSA challenge. Both SrcY527F and SrcY527F/K295M elevated the basal level of membrane ER46. In these two transfectants, E2BSA-induced ER46 recruitment was not observed, likely because membrane ER46 levels were already near maximal. Furthermore, the changes of membrane ER46 expression conferred by wild-type Src or Src mutants correlate with the ability of ER46 to bind membrane-impermeant ligands, determined by HRP activity associated with membrane ER46 after E2HRP (membrane-impermeant) treatment of reconstituted COS-7 (Fig. 5B), SYF−/− (Fig. 5C), and YF−/−, Src+/+ (Fig. 5D) cells in culture. SrcY527F and SrcY527F/K295M increased (2.4 ± 0.2-fold and 2.6 ± 0.1-fold, respectively) membrane binding of E2HRP. Our data suggest that acquisition of an open conformation of c-Src enables functional presentation of ER46 in the plasma membrane.
Fig. 5.
Adaptation of membrane ER46 with open conformation of c-Src. (A) Requirement of open conformation of c-Src for membrane localization of ER46. (B–D) c-Src mediated membrane ER46 binding ligands at cell surface. Values are expressed as means ± SD (n = 4) of the indices [(sample readout/mg of protein)/(untransfected SYF−/− readout)]. *, P < 0.05 (two-tailed ANOVA, sample vs. vector).
Effect of ER46–c-Src Interaction Domains on eNOS Activation.
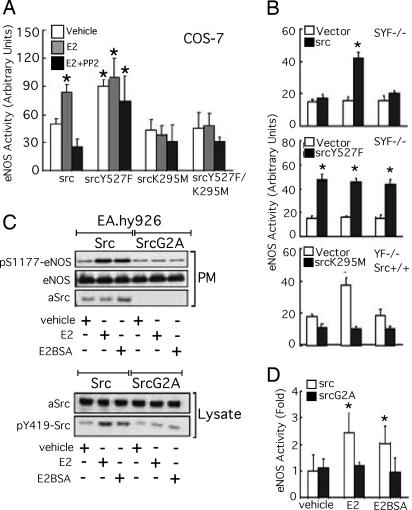
We have demonstrated that c-Src tyrosine kinase activity is important for ligand-stimulated eNOS activation through ER46/phosphatidylinositol 3-kinase/Akt/eNOS signal transduction (3). We thus addressed how c-Src conformation determines c-Src kinase activity in this pathway. In ER46- and eNOS-reconstituted, selected Src-transfected COS-7 cells, E2 elicited a PP2-sensitive eNOS activity increase (1.6 ± 0.3-fold) in wild-type Src transfectants (Fig. 6A). In contrast, neither SrcK295M nor SrcY527F/K295M was able to confer E2-induced eNOS activation, further demonstrating that this pathway requires c-Src kinase activity. SrcY527F provided the cells with constitutively elevated eNOS activity that was neither enhanced nor inhibited by E2 and PP2, respectively. Similar findings were obtained if membrane-impermeant E2BSA was used in ER46- and eNOS-reconstituted SYF−/− and YF−/−, Src+/+ cells (Fig. 6B). The data strongly suggest that the open conformation, although permissive of ER46 interactions that are important for the multimeric complex assembly and membrane localization, cannot override the requirement for tyrosine kinase function and that c-Src kinase activity is mandatory for the E2-induced signaling event. The loss of sensitivity of SrcY527F to PP2 may be caused by unfolding the deep hydrophobic pocket, a tertiary structure essential to PP2 binding to the Src family lck tyrosine kinase (15). Notably, in EA.hy926 cells, compared with heterologous expression of avian wild-type Src, avian SrcG2A mutants, defective in membrane ras activation (16) without affecting its tyrosine kinase activity (17), were unable to transmit E2 or E2BSA signals for c-Src Y419 phosphorylation, eNOS S1177 phosphorylation, and eNOS activation (Fig. 6 C and D). Thus, membrane-initiated E2 signaling is directed through membrane-targeted c-Src.
Fig. 6.
Activation of eNOS signaling by c-Src tyrosine kinase activity. (A and B) Requirement of c-Src kinase activity for eNOS activation. In the presence and absence of 10 μM PP2, transfected cells were treated with 30 nM E2 or E2BSA for 10 min, followed by eNOS activity assessment. Results are plotted as means ± SD (n = 3) of the indices [(sample readout/mg of protein)/(boiled lysate readout/mg of protein)]. *, P < 0.05 (two-tailed ANOVA, treated vs. vehicle). (C and D) Requirement of membrane c-Src kinase activity for eNOS activation in ECs. EA.hy926 cells electroporated with Src or SrcG2A were treated with 30 nM E2 or E2BSA for 10 min, fractionated, immunoblotted, and subjected to eNOS activity assay (n = 3 in D). Lysate immunoblotting (C) was conducted on two different membranes that were transblotted with identical samples.
Discussion
These studies focus on interactions between the ERα splice form, ER46, and the nonreceptor tyrosine kinase c-Src. We believe that their interdependence is critical for mutual membrane localization and transduction of E2-stimulated signals in EC. However, although important and, in fact, required in our system, these two proteins comprise a subset of signaling molecules within a membrane (caveolae)-localized complex that initiate rapid signaling responses to estrogen. Heterotrimeric G proteins including Gαi and Gβγ have been shown to productively interact with ERα and promote E2-stimulated NO release (18, 19). In some models, ERα-dependent induction of eNOS can be driven by activation of matrix metalloproteinases, epidermal growth factor receptor activation, and consequent ERK MAP kinase activation cascades (20). Additionally, rapid responses to nonpeptide hormones other than E2, triggering a phosphatidylinositol 3-kinase/Akt-dependent eNOS activation, have been observed (21). Thyroid hormone can trigger this rapid activation response through engagement of the predominant thyroid hormone receptor in EC, TRα1. Thus, there are clearly multiple potential rapid signaling pathways activated by ER engagement and redundancy of receptor classes that, upon engagement, can facilitate identical outcomes of endothelial activation, namely NO release.
Our studies indicate that c-Src is required for E2-induced vasorelaxation. Based on the inhibition of vasodilation observed when aortic rings were pretreated with L-NAME or a conventional ER antagonist, the induced vascular responses are, at least in part, NO- and ER-dependent. These ex vivo data are entirely consistent with our (and others') prior in vitro findings that E2 triggers an ER-dependent, c-Src/phosphatidylinositol 3-kinase/Akt-mediated activation of eNOS, with consequent EC NO release (2, 3, 12). Recently, E2 has been shown to induce Akt S473 phosphorylation and rapid NO production in isolated cerebral blood vessels (22), also consistent with both our ex vivo and in vitro data. In this work, in addition to demonstrating the vascular tissue correlate of our in vitro data, our efforts were directed at defining molecular interaction domains between ER and c-Src and assessing the importance of classical c-Src structure and function and the interdependence of these two molecules for plasma membrane targeting, all in the context of eNOS activation. Indeed, the molecular determinants of these activation events include an interdependent plasma membrane targeting with a subset of c-Src bearing a substrate-accessible conformation, as well as a function-modifiable Src kinase domain.
The c-Src C-terminal Y530 (avian 527) is an important regulatory phosphorylation site. Under basal conditions, 90–95% of the c-Src molecules are phosphorylated by constitutively active tyrosine kinases such as Csk and Chk (23). The p-Y530 binds intramolecularly to its SH2 domain, generally believed to prevent substrate accessibility and the required Y419 (avian 416) phosphorylation. A key activation switch often occurs when phosphatases such as PTPα dephosphorylate p-Y530 with consequent dissociation from the SH2 binding pocket. In our study, c-Src presents a substrate-accessible conformation without Y530 dephosphorylation (Fig. 2). Instead, protein–protein interactions, perhaps disrupting the intramolecular rigidity without affecting Y530 dephosphorylation (24, 25), may largely contribute to opening c-Src. The availability of a low level of c-Src kinase activity in resting cells (13) and the increasing association of ER46 with E2 stimulation (Fig. 3A) may favor homotypic (26, 27) and heterotypic (24, 25) protein interactions with c-Src and further enforce c-Src “opening.” Indeed, it has been demonstrated that doubly phosphorylated c-Src can be active, such that Y419 phosphorylation can override the p-Y530-directed inhibition (28). The balance of tyrosine kinase (Csk and Chk) and phosphatase (PTPα) activity, in both basal and stimulated states, is partially responsible for the state of Src activation. We are currently investigating this balance in resting and E2-stimulated EC.
A number of posttranslational modifications and protein–protein interactions have been shown to direct ER localization to plasma membranes. These include palmitoylation on C447 (29) and caveolin-1 interaction with ERα S522 (30), the latter promoting targeting to specialized membrane rafts, caveolae, in EC. Furthermore, multiple proteins, including the modulator of nongenomic activity of ER (MNAR) and striatin, serve as scaffold molecules for membrane complexes containing ER with c-Src (31) and Gαi (32), respectively. Here we demonstrate that productive membrane targeting of c-Src (abrogated by the SrcG2A myristoylation mutant) is also important for maximal plasma membrane targeting of ER. We observed a rapid, E2-stimulated, Src- and ER-dependent ER46 phosphorylation. Although we do not map this phospho-site, others have demonstrated that ERα Y537, when inducibly phosphorylated, binds to the c-Src SH2 domain (31). Because the SrcR175L SH2 mutant loses its E2-induced association with ER46 (Fig. 4), it is likely that this Y537 is the critically stimulated phosphorylation/activation site, responsible for triggering rapid signaling cascades, including those leading to eNOS activation and EC NO release.
We have used EC that, in our experimental conditions, express the truncated ERα splice form ER46 (2). We have also used recombinant ER46 expression in heterologous systems to dissect these molecular interactions in vitro. We find that all of the membrane targeting, molecular complex assembly, and rapid activation responses are achievable with ER46 in EC. It is clear that full-length ERα can also localize to endothelial caveolar structures (31), and the predominance of a given isoform in a physiologically or pathologically relevant vascular context is not known. Both full-length and truncated (≈50 kDa) ERs have been coimmunoprecipitated with eNOS from rat cerebral vessels (22). Also, the original ERα gene-deleted mouse (ERαKOChapel Hill), targeted for deletion within exon 1, was reexamined after vascular responses were found to be maintained. Indeed, truncation variants resulting from both splice and internal translation initiation sites within exon 2, ER55 and ER46, were demonstrated in the vasculature of that mouse and were capable of mediating estrogen responses (24, 33, 34).
Our findings are consistent with human vascular endothelial expression of ER46, a receptor isoform that efficiently promotes the aforementioned, multimeric signalsome and, upon engagement by ligand, facilitates the signal transduction cascade, resulting in NO release (2, 3). Given the major efforts directed at evaluating ovarian steroid replacement effects on the cardiovascular status of postmenopausal women, careful dissection of rapid, membrane-initiated, ER-dependent signaling is timely and critical to more carefully targeted future therapies.
Materials and Methods
Materials and Reagents.
The anti-ERα antibody (F-10), all secondary antibodies, and protein A/G-plus reagent were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies were used for c-Src (Ab-1; Oncogene Research Products, La Jolla, CA), avian Src (EC-10; Upstate Biotechnology, Lake Placid, NY), and eNOS (BD Transduction Laboratories, San Jose, CA), whereas rabbit polyclonal antibodies were used for phospho-S1177-eNOS, phospho-Y416-Src, phospho-Y527-Src, and nonphospho-Y527-Src from Cell SignalingTechnology (Beverly, MA). E2, water-soluble E2, PE hydrochloride, acetylcholine chloride, sodium nitroprusside, and L-NAME were purchased from Sigma (St. Louis, MO). LY294002 and PP2 were purchased from Calbiochem (La Jolla, CA), and ICI 182,780 was from Zeneca Pharmaceuticals (Wilmington, DE). All other reagents were purchased from Sigma unless otherwise noted. All cell culture media were purchased from GIBCO/BRL (Gaithersburg, MD).
Plasmids.
ER46 (amino acids 174–595 of ERα) was obtained by PCR amplification with primers containing a BamHI site using hER19Pu as a template. hER19Pu was gift from P. Chambon (Université Louis Pasteur, Strasbourg, France). The amplified fragment was then subcloned into pSG5 or pCMV-Tag3c (Stratagene). Insert for antisense ERα (AsERα) construct was digested by BamHI from hER0Pu, containing human ERα, and then subcloned into pCMV-Tag3c. The numbering of ERα refers to human ERα (Swiss-Prot accession no. P03372). The plasmids containing avian Src mutants such as SrcG2A, SrcW118K, and SrcR175L were prepared with the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) using avian Src/CA10 as a template. Avian Src constructs containing SrcY527F, SrcK295M, and SrcY527F/K295M were obtained from P. Schwartzberg (National Institutes of Health, Bethesda, MD) and J. Brugge (Harvard University, Cambridge, MA). The numbering of avian Src mutants and human Src refers to the sequences of chicken Src (Swiss-Prot accession no. P00523) and human Src (Swiss-Prot accession no. P12931), respectively. Constructs were all verified by sequencing.
Cell Culture and Transfection.
Murine embryonic fibroblasts derived from embryos deficient in c-Src, Yes, and Fyn (SYF−/−) or fibroblasts derived from embryos lacking both Yes and Fyn but maintaining normal levels of c-Src (YF−/−Src+/+) were obtained from American Type Culture Collection (Manassas, VA). Cells were subcultured and maintained as described previously (3, 11). The immortalized vascular EC line EA.hy926 and human umbilical vein ECs were maintained as described previously (3). For pretreatment, cells were E2-deprived for 48 h in phenol-red-free DMEM or M199 containing 15% gelding horse serum and serum-starved for 12 h in phenol-red-free DMEM or M199 supplemented with 0.25% BSA. Cells were transfected by electroporation. Alternatively, Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used according to the manufacturer's guidelines.
Subcellular Fractionation.
Plasma membranes were isolated by a cationic colloidal silica isolation technique (35). Briefly, cells were dissociated in dissociation buffer (GIBCO/BRL), resuspended in 1% cationic colloidal silica in plasma membrane coating buffer [PMCB; 20 mM Mes, pH 5.5/150 mM NaCl/250 mM sorbitol/1 mM Na3VO4/1 mM NaF/protease inhibitor mixture (Roche, Indianapolis, IN)], overcoated in 1 mg/ml polyacrylate in PMCB, mechanically lysed in hypotonic buffer (2.5 mM imidazole, pH 7.0/5 mM EDTA) by a probe sonicator (Fisher, Pittsburgh, PA), mixed with an equal volume of 100% Nycodenz, layered onto 70% Nycodenz, topped with hypotonic buffer, and sedimented at 60,000 × g for 40 min at 4°C. Soluble proteins and plasma membranes were separately collected, dialyzed against PBS for 2 h at 4°C, and recovered in PBS containing 1 mM Na3VO4.
ER46 Phosphorylation by E2 in Vitro.
Cells were disrupted for 4 h at 4°C in lysis buffer (20 mM Tris·HCl, pH 7.4/1% Nonidet P-40/137 mM NaCl/1 mM CaCl2/1 mM MgCl2/0.1 mM Na3VO4). Aliquots of the supernatant containing 200 μg of protein were precleared by 0.4 μg of irrelevant IgG (OKT3) and protein A/G-plus reagent for 1 h at 4°C and were subjected to immunoprecipitation with anti-ERα antibody (F-10) in the presence of protease inhibitor mixture (Roche) overnight at 4°C. Immunocomplexes were then extensively washed in lysis buffer three times and once in wash buffer (10 mM Tris·HCl, pH 7.5/0.3 M sucrose/1% Nonidet P-40/10% glycerol/0.1 mM EDTA/0.1 mM EGTA/1 mM DTT/1 mM Na3VO4), after which the immunocomplexes were resuspended in 20 μl of kinase assay buffer [20 mM Tris·HCl, pH 7.5/5 mM MgCl2/0.2 mM CaCl2/0.5 mM DTT/10 μCi of [γ-32P]ATP (>4,000 Ci/mmol; 5 μM ATP)], with and without 30 nM E2 and inhibitors as described in indicated figure legends. Incubation was maintained at room temperature for 15 min and terminated by the addition of SDS/PAGE sample buffer.
eNOS Activity Assay.
eNOS activity was monitored by l-[3H]citrulline production from l-[3H]arginine as described previously (36). Briefly, protein samples were incubated in reaction buffer [1 mM l-arginine/100 mM NADPH/1 mM tetrahydrobiopterin/0.2 μCi of l-[3H]arginine (>66 Ci/mmol) per reaction] for 15 min at 37°C, separated by Dowex-50W ion-exchange chromatography in 20 mM Hepes (pH 5.5), 2 mM EDTA, and 2 mM EGTA, and the flow-through was used for liquid scintillation counting.
Surface E2-HRP Binding.
Cell monolayer was incubated with 600 nM E2-HRP for 10 min on ice in the presence and absence of 30 μM E2BSA, washed in PBS, homogenized in lysis buffer [20 mM Tris·HCl, pH 7.4/100 mM NaCl/1% Nonidet P-40/1 mM CaCl2/1 mM MgCl2/1 mM Na3VO4/protease inhibitor mixture (Roche)], immunoprecipitated by F10 or OKT3 IgG (0.2 μg/mg of protein), incubated in 50 μl of substrate reagent (BD Pharmingen) at room temperature until color developed, terminated with 50 μl of 1 M H3PO4, and monitored at 459 nm by spectrophotometry.
Ex Vivo Vasomotion.
Vasodilation experiments were performed as described previously (37) using anatomically dissected thoracic aorta rings from PBS-perfused control and Src−/− mice. Control thoracic aorta rings were from either C57BL/6J or Src+/+ littermates, which yielded similar results.
Supplementary Material
Acknowledgments
We thank Dana Brenckle for assistance with the manuscript and all those generously providing key plasmids. This work was supported by National Institutes of Health Grants R01HL 67182 (to J.R.B) and R01AR042927 (to R.B.) and by a grant from the Raymond and Beverly Sackler Foundation (to J.R.B.).
Abbreviations
- E2
17β-estradiol
- EC
endothelial cell
- ER
estrogen receptor
- L-NAME
Nω-nitro-l-arginine methyl ester hydrochloride
- eNOS
endothelial NO synthase
- PE
l-phenylephrine
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- PTP
protein tyrosine phosphatase
- SH
Src homology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704315104/DC1.
References
- 1.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Haynes MP, Bender JR. Proc Natl Acad Sci USA. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SM, Brugge JS. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder R, London E, Brown D. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorge JD, Jakymiw A, Fujita DJ. Oncogene. 2000;19:5620–5635. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 8.Arnold SF, Vorojeikina DP, Notides AC. J Biol Chem. 1995;270:30205–30212. doi: 10.1074/jbc.270.50.30205. [DOI] [PubMed] [Google Scholar]
- 9.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan KB, Bibbins KB, Swedlow JR, Arnaud M, Morgan DO, Varmus HE. EMBO J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Proc Natl Acad Sci USA. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 13.Arnold SF, Melamed M, Vorojeikina DP, Notides AC, Sasson S. Mol Endocrinol. 1997;11:48–53. doi: 10.1210/mend.11.1.9876. [DOI] [PubMed] [Google Scholar]
- 14.Bibbins KB, Boeuf H, Varmus HE. Mol Cell Biol. 1993;13:7278–7287. doi: 10.1128/mcb.13.12.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Kim JL, Newcomb JR, Rose PE, Stover DR, Toledo LM, Zhao H, Morgenstern KA. Structure (London) 1999;7:651–661. doi: 10.1016/s0969-2126(99)80086-0. [DOI] [PubMed] [Google Scholar]
- 16.Marais R, Light Y, Paterson HF, Marshall CJ. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamps MP, Buss JE, Sefton BM. Cell. 1986;45:105–112. doi: 10.1016/0092-8674(86)90542-8. [DOI] [PubMed] [Google Scholar]
- 18.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Proc Natl Acad Sci USA. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 20.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. J Biol Chem. 2005;9:7460–7488. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 21.Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, et al. Proc Natl Acad Sci USA. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Mol Pharmacol. 2005;67:105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 23.Zheng XM, Resnick RJ, Shalloway D. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dramsi S, Dehoux P, Cossart P. Mol Microbiol. 1993;9:1119–1121. doi: 10.1111/j.1365-2958.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Chen JK, Feng S, Dalgarno DC, Brauer AW, Schreiber SL. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- 26.Barker SC, Kassel DB, Weigl D, Huang X, Luther MA, Knight WB. Biochemistry. 1995;34:14843–14851. doi: 10.1021/bi00045a027. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Huang XY, Cole PA. Biochemistry. 2001;40:2004–2010. doi: 10.1021/bi002342n. [DOI] [PubMed] [Google Scholar]
- 28.Sun G, Sharma AK, Budde RJ. Oncogene. 1998;17:1587–1595. doi: 10.1038/sj.onc.1202076. [DOI] [PubMed] [Google Scholar]
- 29.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trantalance A, Visca P, Marino M. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW. Mol Endocrinol. 2005;19:277–289. doi: 10.1210/me.2004-0008. [DOI] [PubMed] [Google Scholar]
- 31.Barletta F, Wong C-W, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Mol Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 32.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RGW, Shaul PW. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 33.Karas RH, Hodgin JB, Kwoun M, Krege JH, Aronovitz M, Mackey W, Gustafsson JA, Korach KS, Smithies O, Mendelsohn ME. Proc Natl Acad Sci USA. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Jr, Lubahn DB, O'Donnell TF, Jr, Korach KS, Mendelsohn ME. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson BS, Schnitzer JE, McCaffery M, Palade GE. Eur J Cell Biol. 1992;58:296–306. [PubMed] [Google Scholar]
- 36.Russell KS, Haynes MP, Caulin-Glaser T, Rosneck J, Sessa WC, Bender JR. J Biol Chem. 2000;275:5026–5030. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- 37.Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, Gratton J-P, Sessa WC. Circ Res. 2002;90:904–910. doi: 10.1161/01.res.0000016506.04193.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.