Abstract
Before fertilization, vertebrate eggs are arrested in meiosis II by cytostatic factor (CSF), which holds the anaphase-promoting complex (APC) in an inactive state. It was recently reported that Mos, an integral component of CSF, acts in part by promoting the Rsk-mediated phosphorylation of the APC inhibitor Emi2/Erp1. We report here that Rsk phosphorylation of Emi2 promotes its interaction with the protein phosphatase PP2A. Emi2 residues adjacent to the Rsk phosphorylation site were important for PP2A binding. An Emi2 mutant that retained Rsk phosphorylation but lacked PP2A binding could not be modulated by Mos. PP2A bound to Emi2 acted on two distinct clusters of sites phosphorylated by Cdc2, one responsible for modulating its stability during CSF arrest and one that controls binding to the APC. These findings provide a molecular mechanism for Mos action in promoting CSF arrest and also define an unusual mechanism, whereby protein phosphorylation recruits a phosphatase for dephosphorylation of distinct sites phosphorylated by another kinase.
Keywords: anaphase-promoting complex, cytostatic factor Cdc2, cyclin B, meiosis, phosphatase
Vertebrate eggs are arrested in metaphase II of meiosis by an activity known as cytostatic factor (CSF) (1). During CSF arrest, the anaphase-promoting complex (APC) is inhibited, preventing degradation of cyclin B and other substrates necessary for maintenance of M phase.
A key component of CSF activity is contributed by Mos, an upstream activator of the MEK/MAPK/p90Rsk pathway (2–5). Inhibition of this pathway causes failure to stably arrest in meiosis II, whereas ectopic Mos expression in the early cleavage embryo (or in Xenopus egg extracts prepared to mimic these early cleavages) induces a CSF-like M phase arrest, inhibiting APC activation.
Recently, the APC inhibitor Emi2 has been implicated as a component of CSF (6–11). Emi2 is a zinc finger-containing protein whose binding to the APC inhibits APC function (12). During CSF arrest, Emi2 is bound to the APC, preventing substrate degradation. Upon fertilization, CaMKII-mediated phosphorylation of Emi2 promotes docking of Polo-like kinase 1 (Plx1 in Xenopus) and subsequent Plx1-mediated Emi2 phosphorylation (13–15). This phosphorylation creates a phosphodegron for the E3 ubiquitin ligase SCFβTrCP, leading to rapid Emi2 degradation, alleviating APC inhibition, and promoting M phase exit into the first embryonic interphase.
Emi2 regulation can be reconstituted in extracts prepared from Xenopus eggs. Inclusion of a calcium chelator in the lysis buffer yields a CSF-arrested extract that can be released into interphase by calcium addition. Analysis of Emi2 in this system has revealed an additional level of regulation distinct from the rapid destruction promoted by CaMKII/Plx1. Because cyclin B synthesis continues during CSF arrest, Cdc2/cyclin B kinase activity could rise unabated if synthesis were not counterbalanced by ongoing slow cyclin B degradation. As we reported recently, this degradation is positively regulated through Cdc2-mediated Emi2 phosphorylation (12, 16). Phosphorylation of Emi2 near its C terminus weakens the Emi2–APC interaction, allowing slow cyclin B degradation. Once cyclin levels and Cdc2 kinase activity recede to a certain threshold, dephosphorylation of Emi2 at the Cdc2 phosphorylation sites restores Emi2 activity, halting further cyclin degradation. These findings suggested the existence of a regulatory loop consisting of Cdc2 and an Emi2-directed phosphatase. Inhibitor and protein interaction studies showed this dephosphorylation to be catalyzed by PP2A.
Despite their importance for CSF activity, it was unclear whether Emi2 and Mos acted independently or worked cooperatively to promote and maintain CSF arrest. Recently, however, it was demonstrated that Mos-activated Rsk phosphorylates Emi2, which somehow promotes Emi2 stability and function (17, 18). We show here that Rsk phosphorylation of Emi2 promotes recruitment of PP2A to Emi2. Further, Rsk phosphorylation does not enhance Emi2 function or stability when Emi2 is mutated to abrogate PP2A binding. PP2A binding to Emi2 modulates not only dephosphorylation of the C-terminal Cdc2 sites to control APC binding, but also a set of previously uncharacterized N-terminal Cdc2 sites that control the slow turnover of Emi2 during M phase arrest. These results suggest that the homeostatic levels, and not simply APC-binding capacity, of Emi2 are actively maintained during CSF arrest. Collectively, these findings provide a mechanistic explanation for the ability of Mos to promote CSF arrest through PP2A-mediated control of both function and stability of Emi2.
Results
Mos-Induced CSF Arrest Requires Emi2.
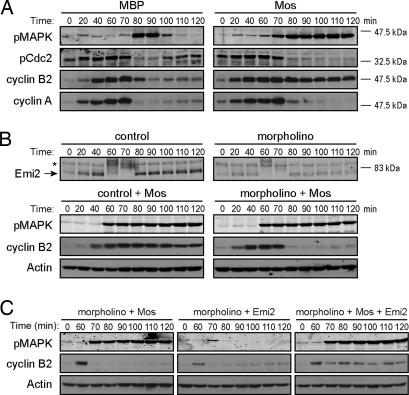
Although Mos levels are low or undetectable during interphase in cycling egg extracts, addition of exogenous Mos can promote an M phase arrest as the extract enters mitosis. This CSF-like arrest is typified by the stabilization of cyclin B, but not cyclin A (Fig. 1A), and Ca2+ addition promotes cyclin B degradation and reentry into the interphase [supporting information (SI) Fig. 5]. Because Mos-induced arrest is manifested as inhibition of the APC, we wished to determine whether this arrest required the APC inhibitor Emi2. Because Emi2 is destroyed before exit from CSF arrest, Emi2 levels are low as the first embryonic interphase begins. Thus, we can treat cycling extracts in this first interphase with morpholino antisense oligonucleotides complementary to the 5′ untranslated region of Emi2 mRNA to inhibit Emi2 reaccumulation (11). The Emi2 morpholinos dampened Emi2 translation without affecting cyclin B accumulation (Fig. 1B and SI Fig. 6). Mos no longer induced M phase arrest when Emi2 levels were decreased, as evidenced by an inability to prevent cyclin B degradation (Fig. 1B). (Note that Emi2 levels were analyzed in separate reactions because our anti-Emi2 antibodies, raised against an MBP fusion protein, strongly cross-react with the MBP-Mos, which migrated at the same gel position as Emi2.) Moreover, readdition of 15-nM Emi2 (a level comparable to that found in CSF extracts) (17) restored the ability of ectopic Mos to induce M phase arrest in Emi2-depleted cycling extracts, whereas readdition of Emi2 alone had no effect (Fig. 1C), confirming recently published reports that Mos exerts its effects through Emi2 (17, 18).
Fig. 1.
Mos requires Emi2 to induce M phase arrest. (A) Cycling extracts were incubated at room temperature for 45 min and were then supplemented with MBP or MBP-Mos. Aliquots were withdrawn at indicated times and immunoblotted for phosphoMAPK (pMAPK), cyclin B2, cyclin A, and phosphoCdc2 (pCdc2). (B) Two sets of cycling extracts in the presence of control or Emi2 antisense morpholinos were incubated at room temperature for 45 min. Mos protein was added to one set of the extracts. Samples were taken at indicated times. Samples taken from extracts without Mos were used to immunoblot for Emi2, and samples taken from extracts with Mos were used to immunoblot for pMAPK and cyclin B2. The asterisk marks a nonspecific band. (C) Cycling extracts were incubated at room temperature in the presence of Emi2 antisense morpholinos. After 45 min, Mos protein, Emi2 protein, or both were added. Samples were taken at indicated times, and pMAPK and cyclin B2 were examined by immunoblotting.
Mos Enhances Emi2-Mediated APC Inhibition by Promoting T545/551 Dephosphorylation.
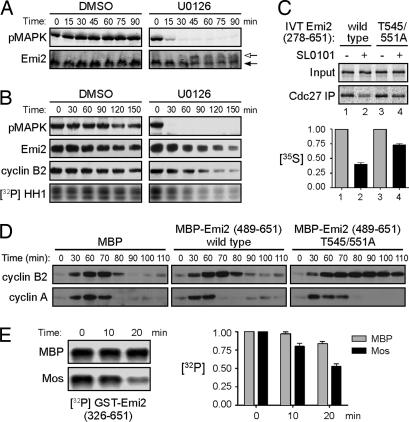
The ability of Emi2 to maintain APC inhibition during CSF arrest can be controlled through Cdc2-mediated phosphorylation at two residues in the C-terminal region of the protein, T545 and T551 (12, 16). Phosphorylation of these sites promotes Emi2 dissociation from the APC, thereby enhancing degradation of APC substrates without affecting Emi2 stability. Because Mos-induced CSF arrest required Emi2, we postulated that Mos might regulate Emi2 phosphorylation to control Emi2–APC interaction. Emi2 in cycling extracts displayed a pronounced shift in its electrophoretic mobility coincident with Cdc2 activation (SI Fig. 7A) (8), which shift was collapsed by λ-phosphatase treatment (SI Fig. 7B). However, we observed that, during Mos-induced M phase arrest, the Emi2 mobility shift was markedly dampened, suggesting that Mos opposes the extensive phosphorylation of Emi2 during M phase (SI Fig. 7A). Supporting this idea, we found that the MEK inhibitor U0126 increased Emi2 phosphorylation in CSF extracts, as indicated by an upshift in Emi2 gel mobility (Fig. 2A and SI Fig. 7C). This mobility shift made it difficult to ascertain whether Emi2's stability had been affected by inhibition of Mos/MAPK signaling. Thus, we treated samples with λ-phosphatase before SDS/PAGE and found that MAPK inhibition partially destabilized Emi2 and also promoted CSF exit (Fig. 2B).
Fig. 2.
Mos regulates Emi2 T545/551 dephosphorylation. (A) CSF extracts were incubated at room temperature in the presence of DMSO or 200 μM U0126. Samples were taken at the indicated times and were immunoblotted for pMAPK and Emi2. Arrows indicate Emi2 mobility shift. (B) CSF extracts were incubated at room temperature in the presence of DMSO or 250 μM U0126. At the indicated times, samples were withdrawn to examine pMAPK and cyclin B2. Samples were also taken to examine endogenous Emi2 after λ-phosphatase treatment and to measure Cdc2/cyclin B kinase activity. (C) (Upper) CSF extracts were supplemented with 40 nM nondegradable cyclin B1. After 90 min, IVT 35S-labeled Emi2 was added in the presence or absence of Rsk inhibitor, SL0101. After 30 min of incubation, Cdc27 was immunoprecipitated and washed, and bound Emi2 was examined by SDS/PAGE and autoradiography. (Lower) The amount of 35S-labeled Emi2 bound to Cdc27 was quantified, normalized, and plotted. Error bars represent the standard deviation of three replicates. (D) Cycling extracts were incubated at room temperature in the presence of MBP, MBP-Emi2 (489–651) WT, or MBP-Emi2 (489–651) T545/551A proteins. Samples were taken at indicated times, and levels of cyclins A and B2 were examined by immunoblotting. (E) (Left) GST-Emi2 (326–651) bound to glutathione Sepharose was phosphorylated in vitro with Cdc2/cyclin B and [γ-32P]ATP and then incubated in interphase extracts pretreated with MBP or MBP-Mos. At indicated times, beads were washed, and the remaining phospho-Emi2 was examined by SDS/PAGE and autoradiography. (Right) The amount of 32P-labeled Emi2 remaining was quantified, normalized, and plotted. Each column represents the average of three replicates, and error bars represent the standard deviation.
To address Mos/MAPK effects on phospho T545/551-regulated Emi2 activity independent of effects on Emi2 stability, we used a nondegradable fragment of Emi2. Inhibition of the Mos-activated kinase Rsk by SL0101 (18) promoted partial dissociation of Emi2 from the APC in CSF extracts (Fig. 2C). APC binding of Emi2 lacking the Cdc2 phosphorylation sites (T545/551A) was noticeably less affected by Rsk inhibition, supporting the notion that Mos/MAPK signaling promotes cyclin stability at least in part through opposing Cdc2-induced Emi2–APC dissociation (Fig. 2C). Moreover, 15-nM Emi2 T545/551A was sufficient to cause M phase arrest in cycling extracts even without Mos addition, whereas wild-type (WT) Emi2 was unable to do so at this dose (Fig. 2D) (a nondestructible Emi2 fragment was used in this experiment to circumvent the Mos requirement for Emi2 stability during prolonged mitotic arrest, as described further in Fig. 3). As observed for the Mos-induced mitotic arrest, the Emi2 T545/551A-induced arrest was typified by stabilization of cyclin B, but not cyclin A (Fig. 2D).
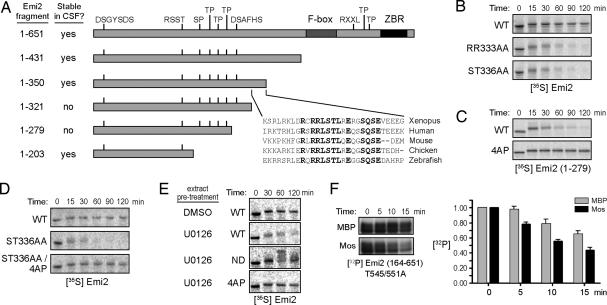
Fig. 3.
A group of Cdc2 sites controlling slow Emi2 degradation are dephosphorylated in response to Mos/MAPK signaling. (A) A series of Emi2 deletion mutants was made to examine the stability of radiolabeled Emi2 fragments in CSF extracts. Constructs listed as stable in CSF extracts were as stable as full-length Emi2. The 1–321 and 1–279 proteins were unstable (SI Fig. 6A). The sequence of Xenopus Emi2 (amino acids 322–350) was aligned with four other species, and the conserved residues are highlighted. (B) Indicated radiolabeled IVT Emi2 proteins were added to CSF extracts and incubated at room temperature. Samples were taken at indicated times and examined by SDS/PAGE and autoradiography. (C) Indicated Emi2 proteins were processed as in B. (D) Indicated Emi2 proteins were processed as in B. (E) Radiolabeled IVT Emi2 (WT, 4AP, or ND) proteins were added into CSF extract pretreated for 30 min at room temperature with DMSO or 250 μM U0126. Samples were taken at indicated times after IVT protein addition, and 35S-labeled Emi2 was detected by SDS/PAGE and autoradiography. (F) GST-Emi2 (164–651, T545/551A) protein was processed as in Fig. 2E except that each column represents the average of four replicates.
In principle, Mos could oppose T545/551 phosphorylation either by retarding Cdc2-mediated phosphorylation of these residues or enhancing their dephosphorylation. We did not observe any effects of Mos on the activity of Cdc2 toward Emi2's C terminus (data not shown). To examine the effects of Mos on dephosphorylation of these sites, we prephosphorylated a large fragment of Emi2 (amino acids 326–651) by using Cdc2/cyclin B and radiolabeled ATP. This fragment contains no other S/T-P sites, and Cdc2 does not phosphorylate the T545/551A mutant in vitro (data not shown). The radiolabeled GST-Emi2 was added to the interphase egg extracts supplemented with either MBP or MBP-Mos. Addition of Mos to extracts significantly accelerated Emi2 dephosphorylation at T545/551 (Fig. 2E). Collectively, these data suggest that Mos promotes CSF arrest, at least in part, by keeping Emi2 dephosphorylated and bound to the APC.
Mos Enhances Dephosphorylation of Multiple Cdc2 Sites That Control Emi2 Stability.
Although Mos promoted Emi2 activity, we also observed that Emi2 stability was affected by MAPK inhibition in CSF extracts (Fig. 2B). An established series of biochemical events (CaMKII and Plx1 phosphorylation, recognition by βTrCP) promotes rapid Emi2 degradation during Ca2+-mediated release from CSF arrest. However, the signals regulating the slow degradation of Emi2 during mitotic arrest that could potentially be targeted by Mos have not been established.
To identify regions of Emi2 regulating its stability during CSF arrest, we produced a panel of in vitro translated (IVT), radiolabeled Emi2 deletion mutants and assessed their stabilities in CSF-arrested extracts. Fragments of Emi2 spanning amino acids 1–431 or 1–350 were as stable as full-length Emi2 in CSF extracts (Fig. 3A and SI Fig. 8A). However, fragments spanning amino acids 1–321 or 1–279 were slowly destroyed over a 1- to 2-h period. This slow degradation required the previously mapped N-terminal degron on Emi2; mutation of this degron (DS33AA) restored Emi2 (amino acids 1–279) stability (SI Fig. 8B). When we further deleted the C terminus of Emi2 (amino acids 1–203), the protein became stable again. All fragments still contained 192RSST and 32DSGYSDS motifs for phosphorylation by CaMKII and Plx1 and were rapidly destroyed upon calcium addition, and all were stable in the interphase extracts (data not shown). These data suggested that a region of Emi2 between residues 204 and 279 might promote Emi2 degradation during prolonged mitoses and raised the possibility that a region between amino acids 322 and 350 could stabilize Emi2 by antagonizing the activity of the 204–279 region.
To determine how the region between amino acids 322 and 350 might affect Emi2 stability, we mutated several conserved residues (Fig. 3A). Mutating RR332/333 or ST335/336 to Ala destabilized Emi2 in CSF extracts (Fig. 3B). These data are interesting in light of recent reports suggesting that Rsk can phosphorylate S335/T336 of Emi2 (18) to promote Emi2 stability. The consensus site for phosphorylation by Rsk is R-X-X-S/T, so the RR333AA mutation also might have disrupted Emi2 phosphorylation by Rsk.
Although Cdc2 can modulate Emi2–APC interaction, we also reported that high Cdc2 kinase activity could promote Emi2 destabilization. Therefore, we mutated multiple candidate Cdc2/cyclin B sites between amino acids 204 and 279 within the context of the mitotically unstable amino acids 1–279 fragment and observed their degradation in CSF extracts. Mutation of any of these sites alone somewhat stabilized the Emi2 fragment (SI Fig. 8C). However, combined mutation of all four sites to Ala (S213A, T239A, T252A, and T267A; hereafter referred to as 4AP) effectively stabilized the 1–279 fragment, consistent with the idea that Cdc2-mediated phosphorylation of these sites might favor Emi2 destabilization (Fig. 3C). Indeed, artificially elevating Cdc2 kinase activity through the addition of cyclin B to the extract promoted slow Emi2 degradation, and this process was abrogated by the 4AP mutation (SI Fig. 8D). Importantly, when the 4AP mutation was examined in the context of full-length Emi2 containing mutations at 335/336, we found that the 4AP mutation compensated for mutation of 335/336, stabilizing Emi2 even in the absence of Rsk-mediated phosphorylation (Fig. 3D). These data raised the possibility that Rsk phosphorylation promotes Emi2 stability by protecting the 204–279 region of Emi2 from phosphorylation by Cdc2.
To further assess the involvement of the Mos/MAPK pathway in controlling Emi2 stability through the 213/239/252/267 sites, we analyzed the effects of MAPK pathway inhibition on the stability of WT and 4AP proteins. Treatment of CSF extracts with U0126 destabilized Emi2, and this effect was abrogated in the 4AP mutant (Fig. 3E). Again this destruction occurred through the known phosphodegrons for βTrCP recognition, presumably after phosphorylation by Plx1, because mutation of DS33 and DS284 to Ala [nondegradable Emi2 (ND)] prevented U0126-induced Emi2 degradation. The Emi2 degron mutant also allowed visualization of more highly phosphorylated forms of Emi2 (Fig. 3E and SI Fig. 8B), which are normally seen after extensive phosphorylation by Plx1 during calcium-dependent Emi2 destruction (6). These highly phosphorylated forms of Emi2 were not seen with the 4AP mutant, suggesting a sequence of events wherein phosphorylation of Emi2 by Cdc2 primes Emi2 for interaction with Plx1, similar to the destruction mechanism for Emi1 (19). These data suggest that the Mos/MAPK/Rsk pathway regulates Emi2 stability through antagonizing phosphorylation at the 213/239/252/267 Cdc2 sites, thereby preventing further phosphorylation by Plx1.
Because Mos activity enhanced dephosphorylation of the 545/551 sites, we hypothesized that Mos also might promote Emi2 stabilization by enhancing Emi2 dephosphorylation, but in this case at the 213/239/252/267 sites. To examine dephosphorylation of these sites in the absence of the 545/551 sites (and also in the absence of additional uncharacterized S-P and T-P sites in the Emi2 N terminus), we phosphorylated a GST-164–651 fragment of Emi2 (T545/551A) by using recombinant Cdc2/cyclin B and [γ-32P]ATP and added this protein to interphase extracts in the presence or absence of supplementary Mos. As shown in Fig. 3F, Mos promoted more rapid dephosphorylation of this Emi2 protein. Thus, Mos may control Emi2 stability during CSF arrest through enhancing the dephosphorylation of 213/239/252/267 sites.
Mos Promotes PP2A–Emi2 Interaction.
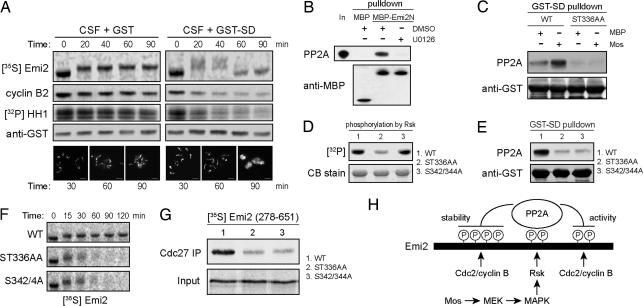
As described previously, deletion analysis revealed that the region of Emi2 containing the Rsk phosphorylation sites was important for regulating Emi2 stability. Indeed, adding an excess of an Emi2 fragment spanning this region [stability domain (SD), amino acids 319–375] to CSF extracts resulted in the failure to maintain CSF arrest as indicated by cyclin B levels, Cdc2 activity, and DNA morphology (Fig. 4A). This exit was accompanied by a greater than usual upshift in Emi2 mobility and some Emi2 destruction before Emi2 returned to its interphase mobility (Fig. 4A). These data suggested that this region might compete with endogenous Emi2 for some factors important for Emi2 activity and stability. Previously, we examined the interactions between Emi2 and PP1 and between Emi2 and PP2A and found that Emi2 could bind to PP2A, but not to PP1 (12). Given our findings implicating Mos in regulating Emi2 dephosphorylation, we postulated that Mos might direct PP2A binding and that phosphorylation of this region by Rsk might mediate this effect. We observed binding of PP2A to the Emi2 319–375 fragment in CSF extract (SI Fig. 9A). Similarly, IVT 1–350 Emi2, but not 1–321 Emi2, coimmunoprecipitated with PP2A from Mos-supplemented interphase extracts (SI Fig. 9B), correlating with our observation that a region within residues 322–350 confers Emi2 stability in CSF extract. Therefore, determinants of PP2A binding to Emi2 lay within the region phosphorylated by Rsk and implicated in Mos control of Emi2.
Fig. 4.
The Mos/MAPK/Rsk pathway promotes interaction of Emi2 with PP2A. (A) CSF extracts were preincubated for 5 min with 0.5 mg/ml GST or GST-Emi2 (amino acids 319–375, SD), after which IVT 35S-labeled Emi2 was added to the extract. Samples were taken at indicated times to detect cyclin B2 and added GST by immunoblotting, Cdc2 kinase activity, and full-length Emi2 tracer by autoradiography. In addition, samples were taken to examine Hoechst-stained nuclei supplemented into the extract. (Scale bars: 25 μm.) (B) MBP or MBP-Emi2N (amino acids 1–350) prebound to amylose resin was added to CSF extracts in the presence of DMSO or 250 μM U0126. After 30 min of incubation at room temperature, the beads were washed, and bound PP2A was examined by immunoblotting. (C) Indicated GST-SD proteins prebound to glutathione Sepharose were incubated in interphase extracts pretreated with MBP or Mos protein. After incubation, beads were retrieved and washed, and bound PP2A was examined by immunoblotting. (D) Indicated GST-SD prebound to glutathione Sepharose was phosphorylated in vitro by using recombinant Rsk and [γ-32P]ATP. After washing, proteins were separated by SDS/PAGE. Proteins were Coomassie blue stained, and incorporated 32P was detected by autoradiography. (E) Indicated GST-SD proteins were incubated in CSF extracts and handled as in B. (F) Indicated radiolabeled IVT Emi2 was added to CSF extracts and incubated at room temperature. Samples were taken at indicated times, and levels of 35S-labeled Emi2 were examined by SDS/PAGE and autoradiography. (G) Indicated radiolabeled IVT Emi2 were mixed into CSF extracts. After 2 h of incubation at 4°C, Cdc27 was immunoprecipitated and washed, and bound IVT Emi2 was examined by SDS/PAGE and autoradiography. (H) Mos-activated Rsk phosphorylates Emi2 at S335 and T336. This phosphorylation promotes Emi2–PP2A association, enhancing Emi2 dephosphorylation at both N- and C-terminal Cdc2 phosphorylation sites. Dephosphorylation of the N-terminal sites promotes Emi2 stability. Dephosphorylation of the C-terminal sites enhances APC-inhibitory binding of Emi2.
If Mos/MAPK signaling acted through PP2A, we expected to see effects of Mos on PP2A binding to Emi2. Indeed, treatment of CSF extracts with U0126 impeded the binding of recombinant Emi2 to endogenous PP2A (Fig. 4B). Collectively, these data strongly suggest that Mos can control Emi2 by regulating binding to PP2A.
Rsk Phosphorylation Affects Emi2 Through Its Ability to Confer PP2A Binding.
Although our data demonstrated that Mos could enhance Emi2–PP2A interaction, they did not demonstrate that this was the primary function of Rsk-mediated Emi2 phosphorylation. Consistent with a role for Rsk phosphorylation in conferring PP2A binding, the ability of Mos to enhance the interaction of the Emi2 319–375 fragment with PP2A in interphase extracts was lost after mutation of the Rsk phosphorylation sites S335 and T336 (Fig. 4C). Proximal to these Rsk phosphorylation sites is a 342SQSE motif conserved among Emi2 orthologues (Fig. 3A). S342 and S344 were recently suggested to be additional sites for phosphorylation by Rsk based on the effect of S342A and S344A mutations on Emi2 gel mobility in CSF extract. However, we found that mutation of S342/344 to Ala had no effect on in vitro phosphorylation of Emi2 by Rsk, but largely abrogated binding to PP2A (Fig. 4 D and E).
This identification of a mutant that retained Rsk phosphorylation, but lacked PP2A binding, afforded us the opportunity to determine whether Mos effects on Emi2 were exerted primarily at the level of PP2A binding. As shown in Fig. 4F, Emi2 ST336AA and Emi2 S342/344A were equally unstable in CSF extract, whereas Emi2 WT protein was stable. We also found that Emi2 ST336AA and Emi2 S342/344A were both markedly impaired in their ability to bind the APC (Fig. 4G). Thus, our data demonstrate that the major function of Mos in CSF arrest is, through Rsk, to promote Emi2–PP2A interaction and thus antagonize phosphorylation of Emi2 by Cdc2/cyclin B. In this manner, Emi2 is kept sufficiently stable and has sufficient APC-inhibitory activity to promote and maintain CSF arrest (Fig. 4H).
Discussion
The arrest of vertebrate eggs in meiotic metaphase II depends on CSF activity, of which the protein kinase Mos is a key component. It is now clear that Mos exerts its effects, in large part, by promoting Rsk-mediated phosphorylation of Emi2. We have shown here that an important function of the Mos/MEK/MAPK/Rsk signaling cascade is to promote the PP2A-mediated dephosphorylation of Cdc2 phosphorylation sites on Emi2 that modulate Emi2 activity and stability.
Maintenance of CSF Arrest Requires Active Mos Signaling.
The ability of Mos to regulate dephosphorylation of sites important for maintaining CSF arrest supports a role for Mos in CSF maintenance. This finding agrees with studies in metaphase II-arrested mouse eggs, in which sustained U0126 treatment caused parthenogenetic activation (20). Previous reports suggesting that MAPK signaling is unnecessary for CSF maintenance in Xenopus extracts used 50 μM U0126 for 30–60 min (21, 22). However, when we used U0126 at higher concentrations (200–300 μM) for longer periods of time (2–3 h), we found that more complete MAPK inhibition in CSF extract in fact caused eventual cyclin B degradation, agreeing with a recent report that immunological neutralization of Mos function also caused spontaneous mitotic exit (23).
It has been reported that CSF extracts immunodepleted of Rsk2 maintain CSF arrest (4). Similar to previous reports using U0126, the depleted extract was only incubated for 60 min, which may not be enough time to allow Emi2–PP2A complex dissociation and Cdc2-dependent Emi2 inactivation. Alternatively, other Rsk isoforms or even unrelated kinases may compensate for the loss of Rsk2 and help maintain Emi2–PP2A interaction. The conservation among vertebrate Emi2 orthologues at the sites for Rsk phosphorylation and PP2A binding (Fig. 3A) suggests that CSF arrest by Emi2 is maintained through a similar mechanism in mammalian systems.
Mos Enhances Emi2 Dephosphorylation.
We found that Cdc2/cyclin B can regulate Emi2 through both C-terminal phosphorylation (T545/551) and a cluster of N-terminal phosphorylation sites. For both the N- and C-terminal sites, Mos accelerated their dephosphorylation. The ability of Mos to enhance PP2A-mediated Emi2 dephosphorylation appears to lie, at least in part, at the level of PP2A–Emi2 binding, in that Mos activity promoted PP2A–Emi2 interaction and mutation of the Rsk phosphorylation sites abrogated this effect. It is also possible that Mos signaling might somehow enhance PP2A's enzymatic activity or dampen the activity of Cdc2/cyclin B during CSF arrest perhaps through activation of Wee1 by MAPK (24).
We also observed background phosphatase activity toward Emi2 in interphase extracts (Figs. 2E and 3F), which could result from residual Mos in the extract. Alternatively, PP2A or another phosphatase may exert some degree of Mos-independent activity toward Emi2. If so, the Mos-dependent recruitment of PP2A to Emi2 during CSF arrest would tip the balance of Emi2-directed kinase and phosphatase activities in favor of dephosphorylation, thereby preserving Emi2 function in the face of constant Cdc2/cyclin B activity. Mos-mediated enhancement of the Emi2–PP2A interaction does not result in complete Emi2 dephosphorylation because Emi2 in CSF or Mos-supplemented cycling extracts still displays a slight retardation in gel mobility. This phosphorylation and its relevance for Emi2 function have not been characterized.
Control of Emi2 Protein Stability.
At the time of fertilization-induced exit from CSF arrest, Emi2 is rapidly degraded. Despite this susceptibility to precipitous degradation, Emi2 is not particularly unstable at other phases of the cell cycle. Phosphorylation of Emi2 at residues S213, T239, T252, and T267 by Cdc2 increases its basal mitotic instability. Thus, the feedback loop, whereby Cdc2 enhances APC activity to permit slow cyclin degradation during CSF arrest, might work by both antagonizing Emi2-APC association and facilitating limited Emi2 turnover.
Importantly, Mos enhanced dephosphorylation of Emi2 at these Cdc2 sites presumably by promoting Emi2–PP2A interaction. Mutation of these Cdc2 sites restored the stability in CSF extract of an Emi2 mutant lacking Rsk phosphorylation sites and thus deficient in PP2A interaction. These data support a model wherein PP2A keeps Emi2 in a stable configuration during CSF arrest, which seems at odds with our previous report that phosphatase inhibition using okadaic acid (OA) promoted Emi2 inactivation, but not destruction, in CSF extracts (13). However, Emi2 inactivation in those experiments was rapid because cyclin B destruction was complete only ≈10 min after OA treatment, whereas Cdc2/cyclin B-driven Emi2 destruction takes 1–2 h to occur (Fig. 3 B–E). We have found that Emi2 is indeed destabilized in OA-treated CSF extracts, provided that cyclin destruction is prevented by APC ectopic inhibition (data not shown).
Addition of excess cyclin B to CSF extracts overrides PP2A's control of both Emi2 activity and stability. The N-terminal Cdc2 sites that regulate Emi2 stability may be less readily phosphorylated (or more readily dephosphorylated) than the C-terminal Cdc2 sites that regulate Emi2 activity. Indeed, excess cyclin B promotes Emi2 inactivation in CSF extracts when added at concentrations (25–50 nM) lower than those required for Emi2 destabilization (>75 nM). This may explain why Emi2 is only partially degraded upon MAPK inhibition or competition of PP2A away from endogenous Emi2 (Figs. 2B, 3E, and 4A). APC-inhibitory activity is the property of Emi2 more sensitive to Cdc2/cyclin B, and the resulting degradation of cyclin B prevents Emi2 from being completely destroyed.
When interphase extracts are pretreated with OA and then induced to enter mitosis by using nondegradable cyclin B, an uncharacterized inhibitor of mitotic proteolysis (IMP) blocks APC activity (25). Because Emi2-mediated CSF arrest is OA-sensitive, rather than OA-dependent, it seems unlikely that Emi2 and IMP are identical.
Mos/Emi2 and the Regulation of M Phase in Embryonic Cells.
In cycling extracts, Emi2 is not precipitously degraded at M phase exit (8). The 10- to 15-min duration of M phase is too brief to permit significant Emi2 degradation through the Cdc2/cyclin B-dependent mechanism described here. It does appear that Cdc2-dependent Emi2–APC dissociation is critical to enable embryonic M phase exit because the level of endogenous Emi2 is sufficient to induce metaphase arrest in the presence of ectopic Mos, and endogenous levels of ectopic Emi2 T545/551A mutant induce M phase arrest even in the absence of Mos. The fact that MAPK levels are elevated in early embryonic mitoses raises the question of why MAPK does not promote arrest through Emi2 in these early M phases. However, because Cdc2 activation temporally precedes MAPK activation (SI Fig. 7A) and there is a lag between activating these kinases (4, 26), the most straightforward possibility is that Cdc2 promotes dissociation of Emi2 from the APC and the APC becomes active before Rsk would phosphorylate Emi2. Thus, MAPK pathway activity may be of significance for Emi2 regulation only in meiotic transitions.
Materials and Methods
Cloning and Protein Expression.
Cloning, expression, and purification of Mos or Emi2 fragments into pMAL-c2X and Emi2 fragments into pGEX-KG were as described (12, 13, 21). GST-Emi2 cloned into pCS2+ was transcribed and translated in vitro by using Promega's (Madison, WI) TNT SP6 high-yield protein expression system.
35S-labeled Emi2 proteins were generated by using the TNT quick-coupled transcription/translation system (Promega) in the presence of radiolabeled Met/Cys (MP Biomedicals, Irvine, CA). Emi2 mutagenesis was performed by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
Protein Analyses.
Antibodies used were rabbit anti-Emi2 (27), mouse anti-cyclin B2 (28), mouse anti-cyclin A (no. 11041; Abcam, Cambridge, MA), mouse anti-PP2A (no. 05–421; Upstate Biotechnology, Lake Placid, NY), rabbit anti-phospho-MAPK (no. 9106; Cell Signaling Technology, Danvers, MA), rabbit anti-phospho-Cdc2 (no. 9111; Cell Signaling Technology), and mouse anti-Cdc27 (no. 9972; Santa Cruz Biotechnology, Santa Cruz, CA).
To examine Rsk-mediated Emi2 phosphorylation, recombinant Rsk2 (Upstate Biotechnology) and glutathione Sepharose-bound GST-Emi2 proteins were incubated in kinase buffer [10 mM Tris·HCl (pH 7.5), 10 mM MgCl2, 1 mM DTT, and 100 μM ATP] in the presence of 5 μCi [γ-32P]ATP at room temperature for 30 min. The beads were washed in PBS plus 300 mM NaCl and 0.1% Triton and eluted with SDS/PAGE sample buffer. [32P]Emi2 was detected by phosphorimager.
For dephosphorylation, GST-Emi2 proteins were prebound to glutathione Sepharose beads and incubated with recombinant Cdc2/cyclin B (New England Biolabs, Ipswich, MA) in kinase buffer in the presence of 25 μCi [γ-32P]ATP at room temperature for 30 min. The beads were washed [10 mM Hepes (pH 7.7), 250 mM sucrose, 2.5 mM MgCl2, 1 mM DTT, and 50 mM KCl] and incubated in interphase extracts pretreated with either MBP or Mos. Aliquots were retrieved, and the beads were washed with PBS plus 300 mM NaCl and 0.1% Triton, eluted with SDS/PAGE sample buffer, resolved by SDS/PAGE, and detected by phosphorimager.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 GM67225 (to S.K.) and R01 GM073023 (to P.K.J.) and by Genentech.
Abbreviations
- APC
anaphase-promoting complex
- CSF
cytostatic factor
- IMP
inhibitor of mitotic proteolysis
- IVT
in vitro-translated
- OA
okadaic acid
- SD
stability domain.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707537104/DC1.
References
- 1.Masui Y, Markert CL. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 2.Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 3.Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt RR, Ferrell JE., Jr Science. 1999;286:1362–1365. doi: 10.1126/science.286.5443.1362. [DOI] [PubMed] [Google Scholar]
- 5.Gross SD, Schwab MS, Lewellyn AL, Maller JL. Science. 1999;286:1365–1367. doi: 10.1126/science.286.5443.1365. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU. Genes Dev. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR, III, Jackson PK. Proc Natl Acad Sci USA. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Grimison B, Lewellyn AL, Maller JL. J Biol Chem. 2006;281:34736–34771. doi: 10.1074/jbc.M606607200. [DOI] [PubMed] [Google Scholar]
- 9.Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. J Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohe M, Inoue D, Kanemori Y, Sagata N. Dev Biol. 2007;303:157–164. doi: 10.1016/j.ydbio.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, et al. Curr Biol. 2007;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen DV, Tung JJ, Jackson PK. Proc Natl Acad Sci USA. 2006;103:608–613. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Maller JL. Curr Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- 16.Hansen DV, Pomerening JR, Summers MK, Miller JJ, Ferrell JE, Jr, Jackson PK. Cell Cycle. 2007;6:732–738. doi: 10.4161/cc.6.6.3937. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama T, Ohsumi K, Kishimoto T. Nature. 2007;446:1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- 18.Inoue D, Ohe M, Kanemori Y, Nobui T, Sagata N. Nature. 2007;446:1100–1104. doi: 10.1038/nature05688. [DOI] [PubMed] [Google Scholar]
- 19.Hansen DV, Loktev AV, Ban KH, Jackson PK. Mol Biol Cell. 2004;15:5623–5634. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips KP, Petrunewich MA, Collins JL, Booth RA, Liu XJ, Baltz JM. Dev Biol. 2002;247:210–223. doi: 10.1006/dbio.2002.0680. [DOI] [PubMed] [Google Scholar]
- 21.Reimann JD, Jackson PK. Nature. 2002;416:850–854. doi: 10.1038/416850a. [DOI] [PubMed] [Google Scholar]
- 22.Tunquist BJ, Schwab MS, Chen LG, Maller JL. Curr Biol. 2002;12:1027–1033. doi: 10.1016/s0960-9822(02)00894-1. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto TM, Iwabuchi M, Ohsumi K, Kishimoto T. Dev Biol. 2005;279:345–355. doi: 10.1016/j.ydbio.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Walter SA, Guadagno SN, Ferrell JE., Jr Mol Biol Cell. 2000;11:887–896. doi: 10.1091/mbc.11.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorlaufer E, Peters JM. Mol Biol Cell. 1998;9:1817–1831. doi: 10.1091/mbc.9.7.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guadagno TM, Ferrell JE., Jr Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- 27.Tung JJ, Padmanabhan K, Hansen DV, Richter JD, Jackson PK. Cell Cycle. 2007;6:725–731. doi: 10.4161/cc.6.6.3936. [DOI] [PubMed] [Google Scholar]
- 28.Casaletto JB, Nutt LK, Wu Q, Moore JD, Etkin LD, Jackson PK, Hunt T, Kornbluth S. J Cell Biol. 2005;169:61–71. doi: 10.1083/jcb.200411056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.