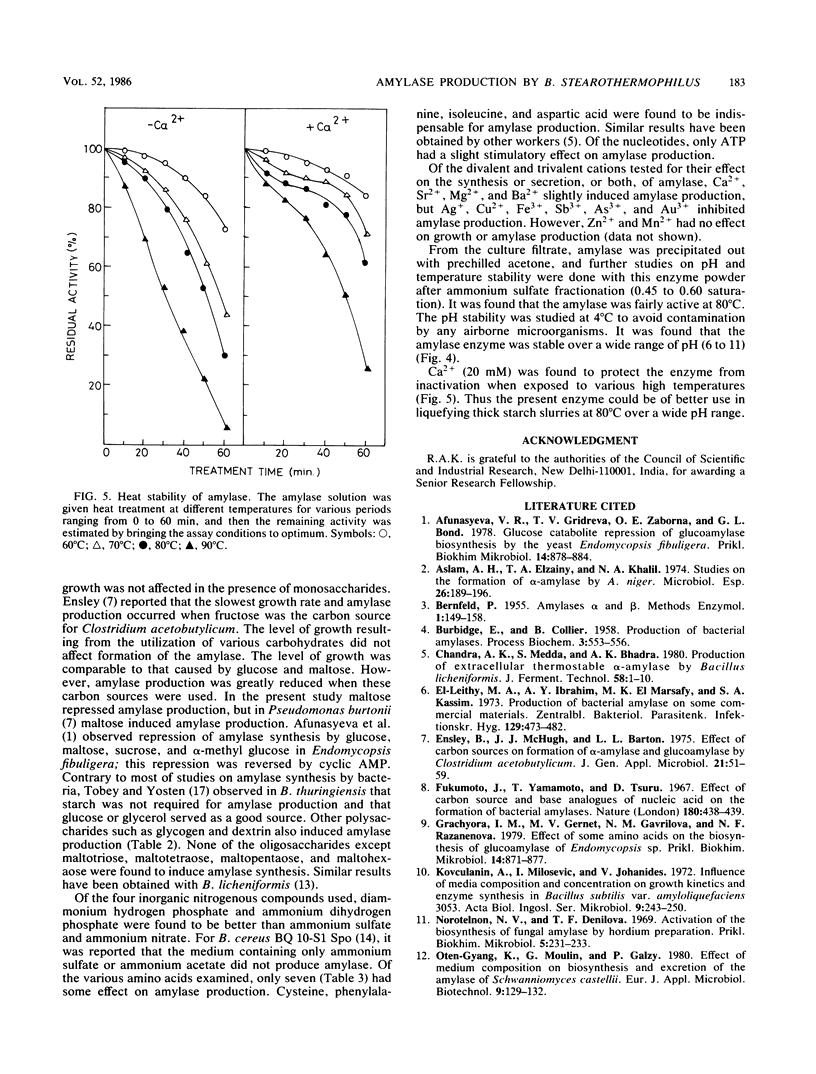
Abstract
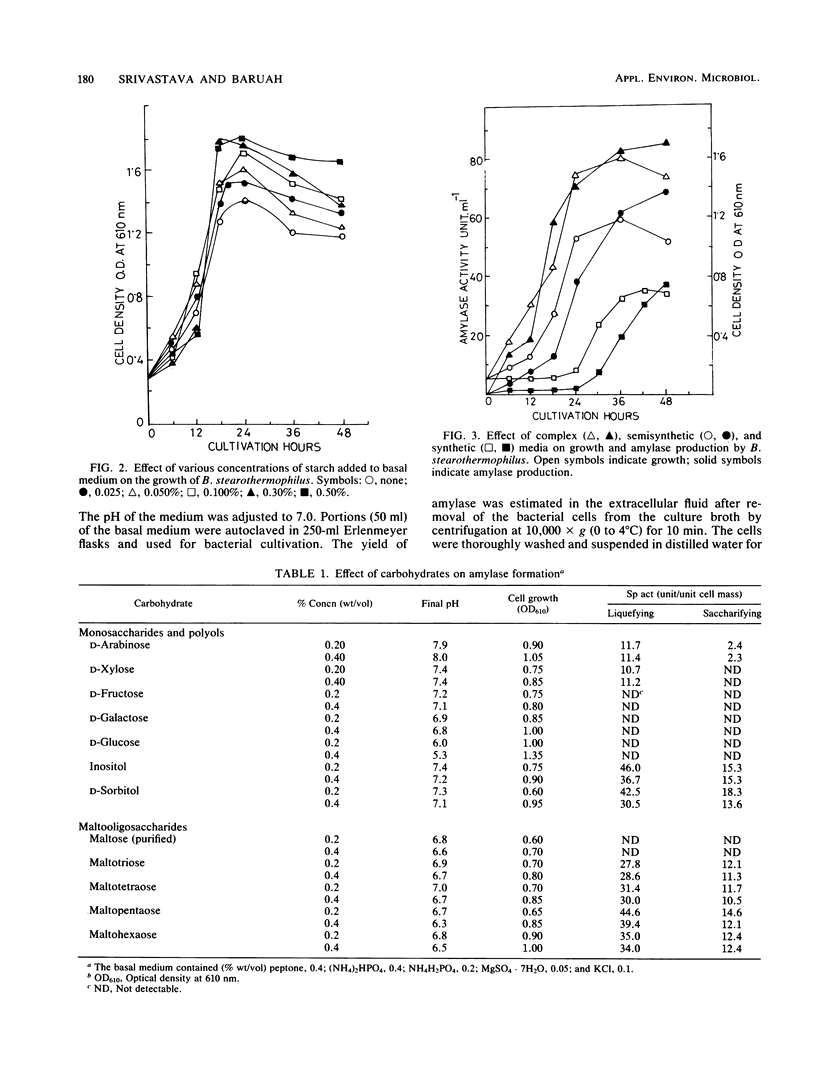
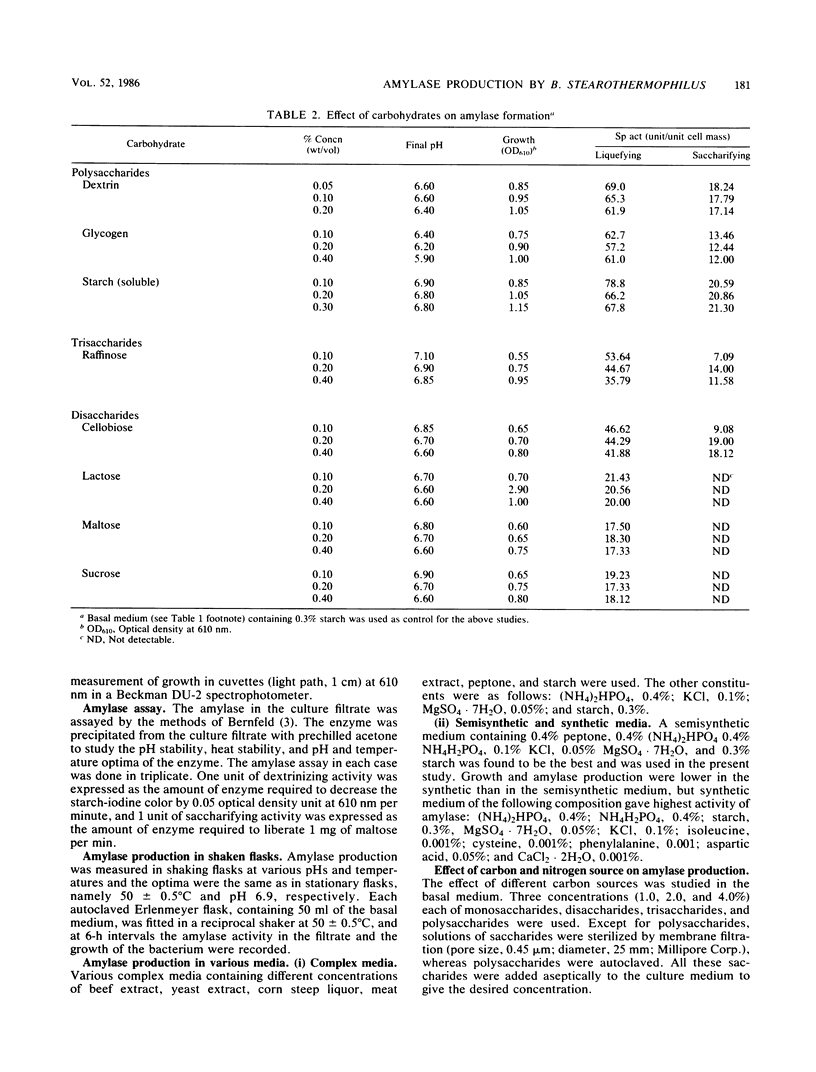
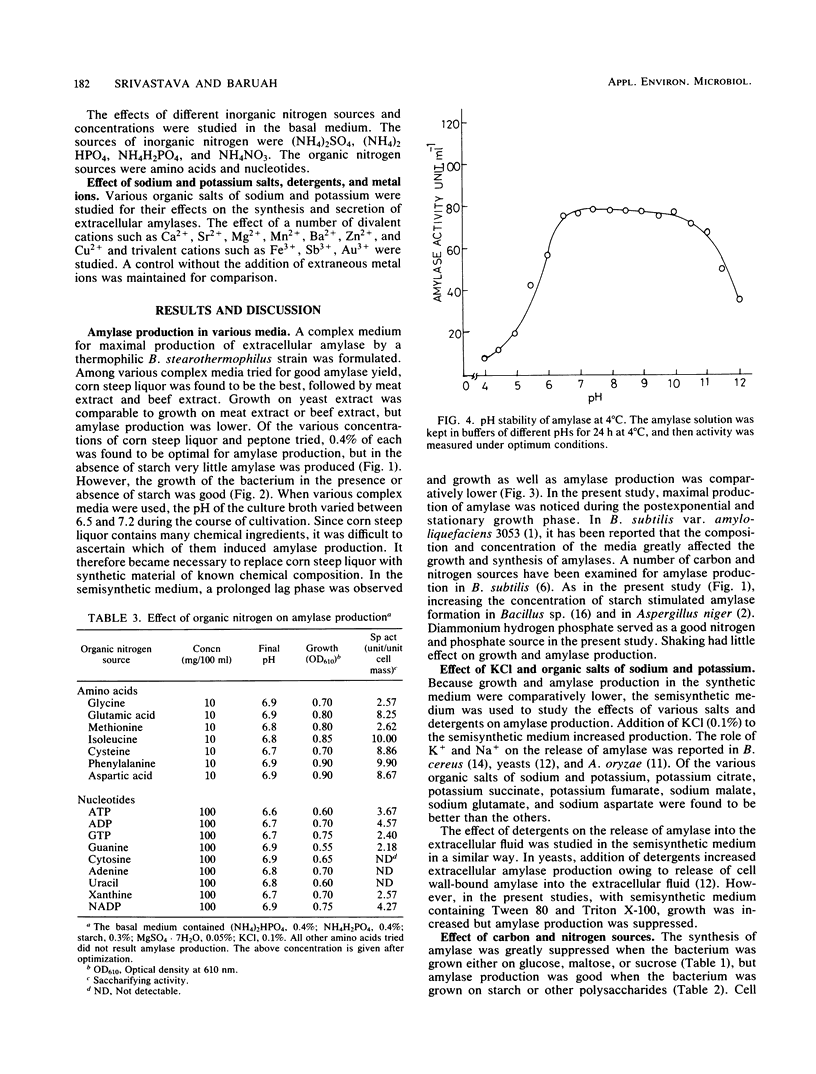
Bacillus stearothermophilus grew better on complex and semisynthetic medium than on synthetic medium supplemented with amino acids. Amylase production on the complex medium containing beef extract or corn steep liquor was higher than on semisynthetic medium containing peptone (0.4%). The synthetic medium, however, did not provide a good yield of extracellular amylase. Among the carbohydrates which favored the production of amylase are, in order starch > dextrin > glycogen > cellobiose > maltohexaose-maltopeptaose > maltotetraose and maltotriose. The monosaccharides repressed the enzyme production, whereas inositol and d-sorbitol favored amylase production. Organic and inorganic salts increased amylase production in the order of KCI > sodium malate > potassium succinate, while the yield was comparatively lower with other organic salts of Na and K. Amino acids, in particular isoleucine, cysteine, phenylalanine, and aspartic acids, were found to be vital for amylase synthesis. Medium containing CaCl2 2H2O enhanced amylase production over that on Ca2+ -deficient medium. The detergents Tween-80 and Triton X-100 increased biomass but significantly suppressed amylase synthesis. The amylase powder obtained from the culture filtrate by prechilled acetone treatment was stable over a wide pH range and liquefied thick starch slurries at 80°C. The crude amylase, after (NH4)2SO4 fractionation, had an activity of 210.6 U mg−1. The optimum temperature and pH of the enzyme were found to be 82°C and 6.9, respectively. Ca2+ was required for the thermostability of the enzyme preparation.
Full text
PDF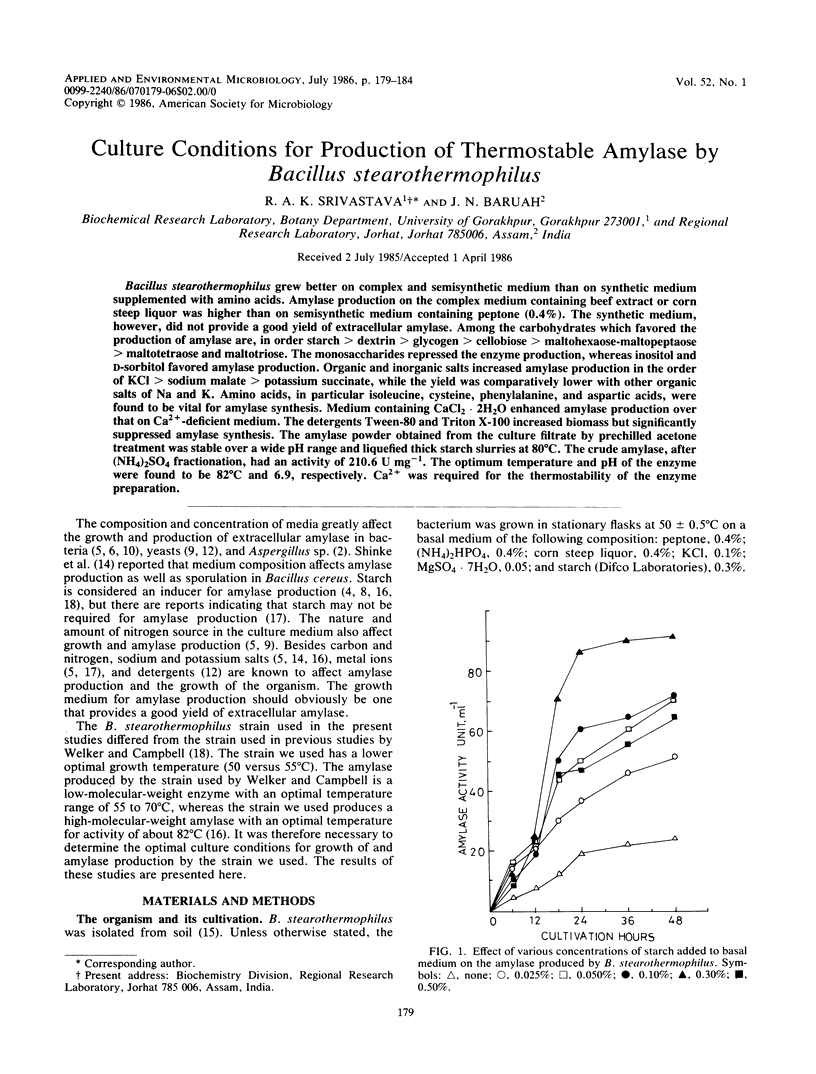

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allam A. M., Elzainy T. A., Khalil N. A. Studies on the formation of alpha-amylase by Aspergillus niger. Microbiol Esp. 1973 Oct-Dec;26(4):189–196. [PubMed] [Google Scholar]
- FUKUMOTO J., YAMAMOTO T., TSURU D. Effects of carbon sources and base analogues of nucleic acid on the formation of bacterial amylase. Nature. 1957 Aug 31;180(4583):438–439. doi: 10.1038/180438b0. [DOI] [PubMed] [Google Scholar]
- Gracheva I. M., Gernet M. V., Gavrilova N. N., Razarenova N. F. Bliianie nekotorykh aminokislot na biosintez gliukoamilazy kul'turoi Endomycopsis species 20-9. Prikl Biokhim Mikrobiol. 1978 Nov-Dec;14(6):871–877. [PubMed] [Google Scholar]
- Saito N., Yamamoto K. Regulatory factors affecting alpha-amylase production in bacillus licheniformis. J Bacteriol. 1975 Mar;121(3):848–856. doi: 10.1128/jb.121.3.848-856.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R. A., Mathur S. N., Baruah J. N. Partial purification and properties of thermostable intracellular amylases from a thermophilic Bacillus sp. AK-2. Acta Microbiol Pol. 1984;33(1):57–66. [PubMed] [Google Scholar]
- Srivastava R. A., Nigam J. N., Pillai K. R., Baruah J. N. Purification, properties & regulation of amylases produced by thermophilic Bacillus Sp. Indian J Exp Biol. 1980 Sep;18(9):972–976. [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Leithy M. A., Ibrahim A. Y., el-Marsafy M. K., Kassim S. A. Production of bacterial amylase on some commercial materials. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1973;128(5):473–482. doi: 10.1016/s0044-4057(73)80067-x. [DOI] [PubMed] [Google Scholar]


