Abstract
Background and purpose:
Activation of P2X receptors on macrophages is an important stimulus for cytokine release. This study seeks evidence for functional expression of P2X receptors in macrophages that had been only minimally activated.
Experimental approach:
Whole-cell recordings were made from macrophages isolated 2–6 h before by lavage from mouse peritoneum, without further experimental activation. ATP (1–1000 μM) elicited inward currents in all cells (holding potential −60 mV). The properties of this current were compared among cells from wild type, P2X1 −/− and P2X4 −/− mice.
Key results:
Immunoreactivity for P2X1 and P2X4 receptors was observed in wild type macrophages but was absent from the respective knock-out mice. In cells from wild type mice, ATP and αβmethyleneATP (αβmeATP) evoked inward currents rising in 10–30 ms and declining in 100–300 ms: these were blocked by pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, 10 μM). ATP also elicited a second, smaller (∼10% peak amplitude), more slowly decaying (1–3 s) at concentrations ⩾10 μM: this was resistant to PPADS and prolonged by ivermectin. Macrophages from P2X1 −/− mice responded to ATP (>100 μM) but not αβmeATP: these small currents were prolonged by ivermectin. Macrophages from P2X4 −/− mice responded to ATP and αβmeATP as cells from wild type mice, except that ATP did not evoke the small, slowly decaying component: these currents were blocked by PPADS.
Conclusion:
Mouse peritoneal macrophages that are minimally activated demonstrate membrane currents in response to ATP and αβmeATP that have the predominate features of P2X1 receptors.
Keywords: P2X1 receptors, P2X4 receptors, knockout mice, macrophages, ATP, PPADS
Introduction
Adenosine 5′-triphosphate (ATP) is an important extracellular signalling molecule and its release evokes a variety of physiological responses in many different tissues and cell types. Its biological effects are mediated by P2 receptors, either the nucleotide-gated ion-channel (P2X) or G-protein-coupled (P2Y) receptor (Ralevic and Burnstock, 1998). Seven mammalian P2X receptor subunits (P2X1–P2X7) have been identified and mRNA for P2X1, P2X4 and P2X7 receptors have been reported in immune cells, such as monocytes, macrophages and brain microglia (North, 2002). In the course of an inflammatory response, high levels of ATP can be released from sources such as platelets, activated leukocytes and dying cells, and ATP is able to induce the release of interleukin-1β (Perregaux and Gabel, 1994). Abundant evidence now indicates that the P2X7 receptor is critical for this IL-1β releasing action (Perregaux and Gabel, 1994; Ferrari et al., 1997; Laliberte et al., 1999; Sanz and Di Virgilio, 2000; Solle et al., 2001). However, macrophages must be primed by interferon-γ or lipopolysaccharide (LPS) before they respond to ATP acting at P2X7 receptors (Perregaux and Gabel, 1994). LPS treatment induces the expression of several hundred genes (Hume et al., 2002). In contrast, little is known about the complement of P2X receptors of resident macrophages at their normal or ‘resting' state; under these conditions they do not release cytokines, but they do display a range of phagocytic and chemotactic properties (van Furth, 1992; Takahashi, 2000).
Electrophysiological measurements of P2X receptor currents have mostly dealt with ‘macrophage-derived' cells maintained in culture. Such studies show that ionic currents induced by ATP have the key defining features of P2X7 receptors (Surprenant et al., 1996; Eschke et al., 2002; North, 2002). However, there are several indications that macrophage-like cells express other types of P2X receptor. Visentin et al. (1999) reported currents in microglia that resembled a combination of P2X4 and P2X7 receptors, and Bowler et al. (2003) showed that a rat lung macrophage cell line (NR8383 cells) had currents with all the characteristic of P2X4 receptors; there was no functional evidence for P2X7 receptors. Coutinho-Silva et al. (2005) reported P2X4- and P2X7-like currents in J774 cells and mouse peritoneal macrophages cultured for 24–48 h.
Recognizing that the expression of ion channels will likely change markedly depending on the level of activation or days in culture, our aim in the present work was to study resident macrophages as soon as practicable after isolation and identification. We used peritoneal macrophages obtained with minimal experimental manipulation, and following pre-incubation with apyrase, we recorded ATP-induced currents within 2–6 h. Given the limited pharmacological tools available with which to characterize P2X receptors (Gever et al., 2006), we supplemented that approach to the identification of subtypes by comparing the properties of cells removed from wild-type mice with those taken from P2X1 (Mulryan et al., 2000) and P2X4 (Sim et al., 2006) knockout mice. The results indicate that the predominant P2X receptor mediating ionic current under these conditions is the P2X1 receptor, with smaller component of P2X4 receptors; this is in contrast to previous studies on activated macrophages.
Materials and methods
Preparation of mouse peritoneal macrophages
Mice used in these studies were C57BL/6J (wild type), P2X1−/− mice (Mulryan et al., 2000) and P2X4−/− mice (Sim et al., 2006). All experiments were carried out under the Animals (Scientific Procedures) Act 1986. Peritoneal macrophages were obtained from mice (26–36-day old) killed with excess isofluorane. The peritoneal cavity was gently lavaged with phosphate-buffered saline (PBS, Invitrogen Life Technologies, Paisley, UK). After centrifugation (280 g for 4 min, DBA-12, Hettich Zentrifugen GmbH, Tuttlingen, Germany), the pellet was resuspended in the recording buffer (mM): NaCl, 147; KCl, 3; MgCl2, 1; CaCl2, 2; HEPES, 10 and D-glucose, 13 (pH adjusted to 7.4 with NaOH) containing apyrase (2 U ml−1; Type VII, Sigma-Aldrich, Poole, Dorset, UK). Cells were plated onto 13 mm glass coverslips in 24-well plates as 100 μl droplets and allowed to settle for 1 h at 37 °C. Each chamber was then flooded with 500 μl PBS containing apyrase and returned to the incubator. Immunocytochemistry and whole-cell recordings were carried out at least 2 h after removal from peritoneal cavity.
Immunocytochemistry and fluorescence microscopy
Macrophages on coverslips were washed once with PBS, fixed with Zamboni's fixative for 15 min at room temperature, and then washed (3 × 10 min) with PBS. For F4/80 staining, cells were blocked with 5% donkey serum in PBS containing 0.3% Triton X-100 for 1 h at room temperature before incubating with rat anti-mouse F4/80 antibody (1:1000; Serotec Ltd, Oxford, UK) for 18–20 h at 4 °C. Cells were washed with PBS (3 × 10 min) and incubated with Cy3-conjugated donkey anti-rat secondary antibody (1:200; Jackson Immuno Research Europe Ltd) for 2 h at room temperature, then rinsed in PBS and mounted with Vectorshield (Vector Laboratories Ltd, Peterborough, UK). In some experiments, we used Vectorshield mounting medium with DAPI to stain nuclei (Vector Laboratories Ltd, UK). For P2X receptor immunocytochemistry, cells were blocked with 5% goat serum in PBS containing 0.3% Triton X-100 (1 h) and incubated with primary antibodies (for P2X1 or P2X4 1:1000; for P2X7 1:500; Alomone Labs, Jerusalem, Israel) overnight at 4 °C. Cells were washed with PBS (3 × 10 min) and incubated in FITC-conjugated goat anti-rabbit secondary antibody (1:200, Jackson ImmunoResearch Europe, Ltd, Newmarket, Suffolk, UK) for 2 h at room temperature. Cells were rinsed in PBS (3 × 10 min) before mounting. Negative controls were performed by omitting the primary antibodies. Positive controls of P2X7 immunoreactivity was verified in human embryonic kidney (HEK) 293 cells transfected with mouse P2X7 receptor cDNA (as described previously in Kim et al., 2001). Macrophages were viewed using an Olympus BX40 fluorescent microscope (Olympus Optical Co. Ltd, London, UK) and images acquired using a Hamamatsu Orca 285 camera (Hamamatsu, Hamamatsu City, Japan) controlled by SimplePCI software (Compix Inc., Sewickley, PA, USA).
Whole-cell recording and agonist application
Coverslips with attached macrophages were placed in a recording chamber mounted on the stage of an Axiovert microscope (Carl Zeiss, Göttingen, Germany). Extracellular recording solution containing (mM): NaCl, 147; KCl, 3; MgCl2, 1; CaCl2, 2; HEPES, 10 and D-glucose, 13 (pH adjusted to 7.4 with NaOH) was superfused at a rate of 5.5 ml min−1. Whole-cell recordings were made at room temperature using an EPC9 amplifier, and data collected using Pulse software (HEKA, Lambrecht, Germany). The membrane potential was held at −60 mV. Patch electrodes and puffer electrodes were pulled from glass pipettes (Harvard Apparatus, Edenbridge, UK) on a vertical puller (HEKA, Lambrecht, Germany) and ranged from 6 to 12 MΩ in resistance when filled with an intracellular solution containing (mM): NaCl, 147; HEPES, 10; EGTA, 10, (pH adjusted to 7.3 with NaOH). ATP and other nucleotides were prepared from 100 mM frozen stock solution (pH adjusted to 7.3) and applied via a glass ‘puffer' pipette (1 μm tip diameter, ≈10 psi, 69 kPa) using a pneumatic PicoPump (PV830, World Precision Instruments, Stevenage, Herts, UK). The tip of the puffer pipette was positioned downstream from the cell with respect to the direction of flow of the superfusing solution, and temporarily repositioned to a point about 15 μm from the cell only for the period of application. After a single application of ATP to any macrophage, a subsequent application 2 min later evoked a current that was less than 20% of the first response. Concentration–response relations shown in this study were constructed from pooled data, in which ATP (or αβmeATP) was applied only once to one macrophage on each coverslip. Antagonists were applied by superfusion for 5 min (suramin and NF449) and 10 min (PPADS and TNP-ATP) prior to the application of ATP (with antagonist). The inclusion of PPADS with ATP made no difference to its degree of antagonism. The ATP-evoked currents were then compared with those observed in other cells with no antagonist pre-treatment.
In some experiments, recordings were made from HEK 293 cells transiently transfected with either mouse P2X1 or mouse P2X4 receptor cDNA. The approach was similar to that described previously in Evans et al. (1995) for human P2X1 receptors. ATP was applied as described for macrophages.
Data analysis
Numerical data are means±s.e. of mean, for the number of macrophages tested. Current traces were obtained using Axograph (Molecular Devices, Sunnyvale, CA, USA), Kaleidagraph (Synergy Software, Reading, PA, USA) and Canvas (ACD Systems, British Columbia, Canada) software. Concentration–response curves were fitted using nonlinear regression (curve fitting) programme from Prism 4 (GraphPad software Inc., San Diego, CA, USA) to the means, s.e.m. and n cells tested at each concentration. The agonist concentration required for the half-maximal responses is expressed as the negative log EC50 (pEC50)±s.e. Statistical significance between data were determined using unpaired t-tests (InStat, GraphPad Software Inc.), and differences were considered significant at the level of P<0.05.
Chemicals
ATP, αβ-methylene-ATP, apyrase, suramin, pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), Brilliant Blue G and ivermectin were purchased from Sigma (Dorset, UK). 2′,3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate (TNP-ATP) and 4,4′,4″,4′″-[carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino))]tetrakis-benzene-1,3-disulphonic acid (NF449) were purchased from Tocris (Bristol, UK).
Results
Macrophages from wild-type mice
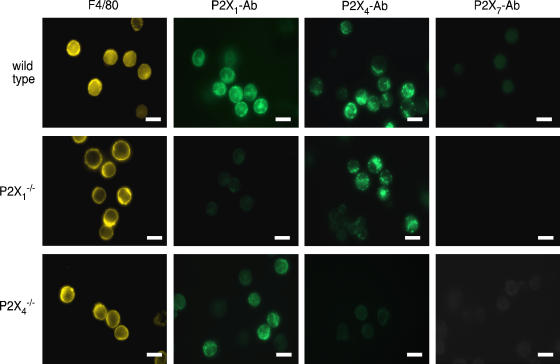
Macrophages from the peritoneal lavage were identified using the macrophage-specific antigen F4/80 (Leenen et al., 1994). Almost (80%) all of F4/80-positive cells (Figure 1) were circular in shape and had kidney-shaped nuclei with DAPI staining: their diameter of 9.8±0.13 μm (n=30). Under phase contrast microscopy combined with epi-fluorescence, the F4/80-positive cells were readily identified as phase bright spherical cells. These cells showed clear P2X1-immunoreactivity and P2X4-immunoreactivity, predominately dispersed in the cytoplasm but with some apparently at the cell membrane. P2X7 receptor staining was not observed (Figure 1) in macrophages under these conditions, although this antibody readily detects P2X7 receptors in rat peritoneal macrophages and other preparations (Kim et al., 2001; Sim et al., 2004). We also observed immunostaining in mouse macrophages treated with lipopolysaccharide (0.1 μg ml−1; 4 h) and then exposed to ATP (1 mM; 5 min; data not shown).
Figure 1.
P2X receptor immunocytochemistry. Leftmost column shows that F4/80 staining was not different in macrophages from wild type or knockout mice. Middle two columns show immunoreactivity for P2X1 and P2X4 receptors in wild type macrophages, whereas they were absent in macrophages from cognate knockout mice. P2X1 immunoreactivity was not obviously altered in cells from the P2X4−/− and vice versa. Right columns show that P2X7 immunoreactivity was undetectable in any peritoneal macrophages, under these conditions (2 h incubation at 37 °C in apyrase after lavage, no LPS). Scale bars 10 μm.
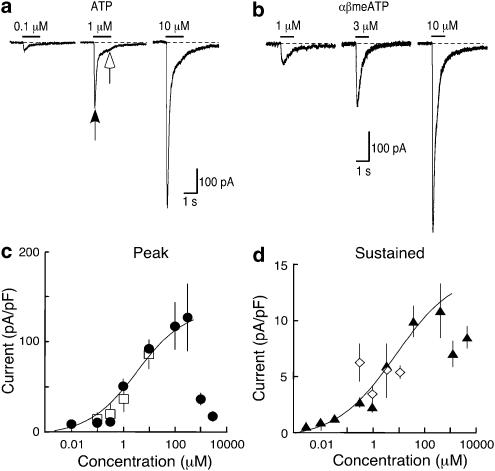
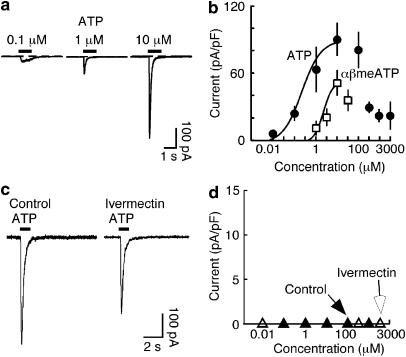
Whole-cell recordings were made from small (<10 μm) circular cells under phase contrast. At a holding potential of −60 mV, these macrophages showed a holding current of −22±1.5 pA and a capacitance of 7.9±0.19 pF (n=150). ATP (1 s application) elicited a current that rose to its peak within a few tens of milliseconds, and then declined during the application (Figure 2a). The decline of the current was clearly biphasic, and best fitted by the sum of two exponentials: the time constants were not different for 10 μM and 100 μM ATP (0.12±0.01 s and 1.9±1.4 s (n=18), 0.10±0.01 s and 2.0±0.33 (n=9) respectively). Concentrations of ATP greater than 100 μM gave much smaller peak currents (Figure 2c), presumably reflecting rapid desensitization. Such fast rising currents in response to ATP were very small or absent in macrophages that had not been incubated with apyrase during the 2–6 h between isolation and recording.
Figure 2.
Currents activated by ATP and αβmeATP in macrophages from wild type mice. (a) Currents shown were evoked by ATP (1 s, concentrations indicated) and (b) by αβmeATP (1 s, concentrations indicated) in three different macrophages in the same animal, respectively. Note the biphasic decay of the current with 1 and 10 μM ATP. Arrowheads indicate amplitudes measured in (d). (c) Peak currents evoked as a function of ATP and αβmeATP concentrations, measured at 200 ms. Note the profound attenuation of the peak current with 1 and 3 mM ATP. (d) Sustained currents measured at 1 s (i.e. at the end of the agonist application) (note different y axis) for ATP and for αβmeATP at the same time point. Each point is mean (±s.e.m.) from pooled data from 5–23 macrophages. Curve fit omits two highest concentrations.
A concentration–response relationship for ATP from pooled data is plotted in Figure 2c, for the peak current and the ‘sustained' current (measured at 1 s); the apparent pEC50 was 5.28±0.25 (n=110 cells). The sustained current at 1 s, about 10% of the peak current, did not decline with the higher ATP concentrations. Similar currents were evoked by αβmeATP (Figure 2). Their rise time was about 80 ms, and they declined with time constants 0.14±0.02 and 0.70±0.07 s (n=14: 10 μM). The concentration–response relation for αβmeATP was similar to that for ATP over the range 100 nM–10 μM (Figure 2b). UTP (100 μM) had no effect (2.1±0.22 pA pF−1, n=5).
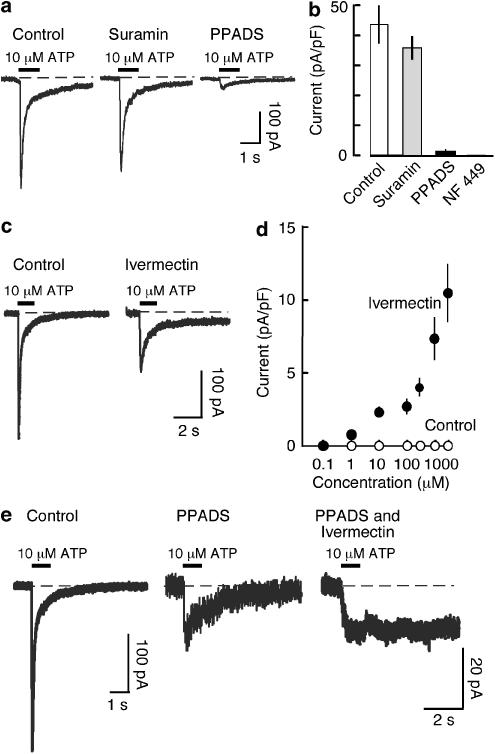
Suramin (10 μM) had little effect on the response to ATP (10 μM) (Figure 3a), whereas PPADS (10 μM) almost completely blocked it. NF 449 (300 nM), an analogue of suramin, completely blocked the effect of ATP (10 μM) (Figure 3b). TNP-ATP (1 μM) inhibited the response to 10 μM ATP by 52.6±6.5 % (n=3) of control. Ivermectin potentiates and prolongs ATP-induced currents at homomeric P2X4 receptors but not at other P2X receptors (Khakh et al., 1999; Priel and Silberberg, 2004): it caused a modest enhancement and noticeable prolongation of the slow component of the ATP-induced current (3 μM; 8 min pre-application). There was also a consistent inhibition of the peak response to ATP (Figure 3c). In ivermectin, the response to a 1 s application of ATP often required several minutes to recover to the baseline zero current. The small residual current observed in the presence of PPADS was also markedly prolonged by ivermectin (Figure 3e).
Figure 3.
Pharmacological properties of currents activated by ATP in macrophages from wild-type mice. (a) Currents evoked by ATP (10 μM, 1 s). Left, control; middle, in suramin (10 μM) and right, in PPADS (10 μM). (b) Histogram shows currents for the four sets of cells indicated (n=4–13). (c) Ivermectin prolongs the slower component of current evoked by ATP (10 μM, 1 s). Ivermectin (3 μM) was applied for 10 min before ATP. (d) Graph shows current amplitude measured at 8 s after ATP application (1 s, concentrations indicated). (e) Ivermectin prolongs residual current in presence of PPADS. Left, control; middle, small current remaining in PPADS and right, small residual current in the presence of PPADS being greatly prolonged by ivermectin.
In parallel control experiments using HEK 293 cells transfected with mouse P2X1 receptor cDNA, we found that suramin (10 μM) had no significant effect on the current evoked by ATP (10 μM), but that NF449 (300 nM) completely antagonized the action of ATP, as seen in the macrophages. Similarly, PPADS (10 μM) almost completely blocked the effect of ATP (10 μM).
Macrophages from P2X1 knockout mice
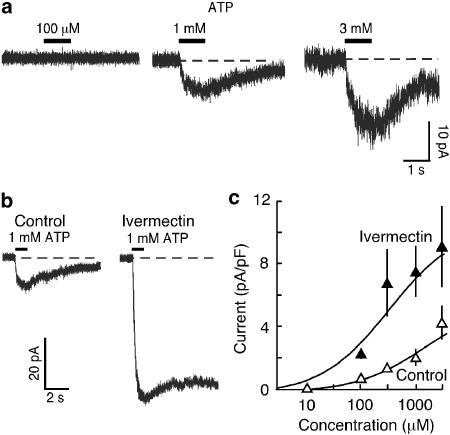
P2X4 immunoreactivity was normal in these macrophages, but no staining was observed with antibodies to P2X1 or P2X7 receptors (Figure 1). The F4/80 positive cells had a diameter (9.9±0.16 μm, n=27), capacitance (7.4±0.27 pF, n=27) and holding current (−24.6±1.9 pA, n=50) not different from wild type cells. These cells showed no response to ATP at 10 μM but higher concentrations (>100 μM to 3 mM, see Figure 4) evoked very small currents (<6 pA pF−1 at 3 mM) that developed slowly (within 1 s) and declined slowly (τ=3.3±0.89 s (n=10) at 1 mM ATP). No responses to αβmeATP were observed in these macrophages. The response to 1 mM ATP evoked in P2X1−/− macrophages was clearly potentiated (∼4-fold) and prolonged by ivermectin (Figure 4b). PPADS (10 μM) slightly potentiated the effect of ATP, whereas Brilliant Blue G (300 nM) had no effect. The effect of PPADS and Brilliant Blue G were confirmed in HEK293 cells transfected with mouse P2X4 receptor cDNA (data not shown). BBG (300 nM) clearly inhibited the responses to ATP evoked in HEK cells transfected with mouse P2X7 receptor cDNA (data not shown).
Figure 4.
Currents activated by ATP in macrophages from P2X1−/− mice. (a) High concentrations of ATP (1 s) were required to elicit current. Each response shown was elicited from different macrophages in the same animal. (b) Small current evoked by ATP (1 mM) was greatly potentiated and prolonged by ivermectin (3 μM). Ivermectin was pre-applied 8 min prior to ATP application in a different cell in the same mouse. (c), Graph shows summary data for effects of ivermectin (3 μM) on ATP-evoked currents. Points are means±s.e.m. from pooled data of 6–8 cells.
Macrophages from P2X4 knockout mice
P2X1 immunoreactivity was normal in these macrophages, but no staining was observed with antibodies to P2X4 or P2X7 receptors. F4/80 positive cells in P2X4−/− mice had mean diameter of 9.9±0.17 μm (n=29). At −60 mV, the holding current (22.7±1.7 pA, n=98) and cell capacitance (7.8±0.17 pF, n=98) were not different from wild type cells (P>0.05). ATP evoked fast-rising and -desensitizing currents that lacked the slowly decaying component observed in wild type macrophages (Figure 5). The current desensitized completely during a 1 s application with a time constant not different from the fast component seen in wild type macrophages (τ=0.14±0.12, n=11; 10 μM ATP). The concentration–response relationship for ATP is shown in Figure 4a, with an apparent pEC50 of 6.44±0.51 (n=82 cells): that is, macrophages derived from P2X4−/− mice were approximately 15-fold more sensitive to ATP than wild type macrophages (P<0.05). On the other hand, αβmeATP also evoked currents in P2X4−/− mice which were not different from those observed in wild type mice (Figure 4a). In PPADS (10 μM), the response to ATP was completely blocked. Ivermectin did not prolong the current evoked by ATP (10 μM), but a reduction in the peak amplitude was observed similar to that seen in macrophages from wild type mice (Figure 5c).
Figure 5.
Currents activated by ATP in macrophages from P2X4−/− mice. (a), Current evoked by ATP (1 s, and concentrations indicated) in three different macrophages obtained from the same mouse, lacked the small slow component on the falling phase, as shown in WT (Figure 2). (b), Graph shows peak current evoked by ATP and αβmeATP. Note profound desensitization with ATP concentrations greater than 300 μM. (c), Ivermectin (3 μM, pre-application 10 min) did not produce any prolongation of the current evoked by ATP (10 μM, 1 s). (d), Graph shows the absence of any current measured 8 s after ATP application, in control or in the presence of ivermectin (3 μM).
Discussion
The macrophages studied in the present experiments were small spherical cells staining homogeneously for F4/80. We interpret this to indicate that they are mature, but minimally activated, peritoneal macrophages. There was no obvious sign of differences in shape, or in cell volume (measured by total cell capacitance) among the macrophages from wild type P2X1−/− or P2X4−/− mice. Preliminary experiments indicated (a) that responses to superfused ATP were extremely variable, and usually absent; (b) that responses to ‘puffs' of ATP were not observed unless the macrophages had been incubated throughout their preparation in apyrase; and (c) that a second application of ATP by ‘puff' application elicited an inward current that was very much smaller in amplitude (about 20%) than the first. We have observed similar properties of mouse P2X1 receptors expressed in HEK cells (JAS and RAN, unpublished observations), and we presume that this reflects the profound desensitization that is reported for other rodent P2X1 receptors (Werner et al., 1996; Rettinger and Schmalzing, 2004). For these reasons, we restricted our ATP applications to a single 1 s ejection from the ‘puffer' pipette, taking precautions to limit any effects of leakage by holding the pipette tip well away from the macrophage, and by studying only a single macrophage on any given coverslip. We also used a sodium-based intracellular solution to reduce any contribution from potassium currents, a variety of which are found in macrophages (Gallin, 1991; Kettenmann et al., 1993).
The rapid-rising, fast-declining inward current that we observed in wild-type macrophages in response to ATP at concentrations greater than 100 nM probably reflects activation of P2X1 receptors. The evidence for this is (a) the current was mimicked by αβmeATP, (b) the current was blocked by PPADS and completely blocked by NF449 and (c) this component of the current was not observed in macrophages for P2X1−/− mice. The lack of effect of suramin was initially surprising, given the effectiveness of the closely related molecule NF449. However, we found that suramin did not antagonize the responses to puff application of ATP (0.1–100 μM) in HEK cells expressing the mouse P2X1 receptors, although NF449 does (JAS and RAN, unpublished observations). We note that Ikeda has recently reported that a similar ATP-induced current in mouse megakaryocytes was insensitive to suramin (Ikeda, 2007), although blocked by the closely related analogue NF023.
It is possible that the slowly decaying component of the current, which we observed in response to ATP in wild-type cells probably results from the activation of P2X4 receptors. This conclusion is supported by the findings that (a) it was not observed with αβmeATP, (b) it was prolonged by ivermectin and (c) a response with similar properties was observed in cells from the P2X1−/− mice. The summation of the currents in response to ATP in cells from the P2X1−/− mice and cells from the P2X4−/− mice fairly closely reproduced the currents observed in the wild type mice. In the present work, the slower component of the ionic current was not blocked by PPADS and nor was the current evoked by a 1 s ‘puff' of ATP (10–100 μM) applied to HEK293 cells transfected with mouse P2X4 subunits (data not shown). On the other hand, Townsend-Nicholson et al. (1999) and Jones et al. (2000) both reported that PPADS inhibited mouse P2X4 receptors expressed in oocytes and HEK293 cells. It is possible that the failure to see antagonism in the present study on account of the non-equilibrium conditions of the very brief ATP application: in the present study we have compared the effectiveness of antagonists such as PPADS between macrophages and HEK293 cells using essentially the same experimental conditions for ATP application. The marked prolongation of the slow component would be consistent with the contribution of P2X4 subunits (Khakh et al., 1999). We found little evidence for currents mediated by P2X7 receptors under these experimental conditions; specifically, there was no effect of Brilliant Blue G (300 nM) at a concentration that can inhibit the response of ATP in HEK cells transfected with mouse P2X7 cDNA (JAS and RAN, unpublished observations). Indeed, our immunocytochemical studies in macrophages under these conditions failed to detect P2X7 protein. The most comparable studies were made on rat (Chen et al., 2005) and mouse (Brough et al., 2003) peritoneal macrophages, where it was concluded that ATP-stimulated calcium influx, ethidium uptake and IL-1β secretion by activating P2X7 receptors. In that case, the effects of ATP were blocked by PPADS (Chen et al., 2005) or observed following LPS treatment (Brough et al., 2003). Ivermectin does not potentiate currents mediated by (rat) P2X7 receptors (Khakh et al., 1999). The lack of any P2X7-receptor involvement in our studies presumably reflects the fact that the cells were minimally activated (other than by plating onto glass coverslips).
Currents mediated by P2X1 receptors have not been reported previously in mouse peritoneal macrophages, and efforts to detect their mRNA have been largely negative (Coutinho-Silva et al., 2005). However, P2X1 receptors have been described in lymphocytes (Sluyter et al., 2001; Wang et al., 2004); indeed a partial P2X1-receptor mRNA was first isolated from thymocytes (Owens et al., 1991). In the rat alveolar macrophage cell line (NR8383), P2X4 receptors have been characterized by RT-PCR, immunocytochemistry and by recording membrane currents that are robustly potentiated by ivermectin (Bowler et al., 2003). The mRNA is also abundant in human monocytes and lymphocytes (Sluyter et al., 2001; Wang et al., 2004).
There was broad agreement between the properties of the macrophages taken from wild type mice, and the sum of the same properties of macrophages taken from P2X1−/− and P2X4−/− mice (for example Figure 5c). However, there were anomalies with respect to the effective concentrations of ATP. In particular, the small, slower current component was elicited by lower concentrations of ATP in the wild type mice (Figures 2 and 3) than in the P2X1−/− mice (Figure 4). The profound desensitization observed at higher concentrations made it difficult to estimate a ‘maximal' current, and thus a log concentration of ATP evoking a half-maximal current (pEC50); the concentration–response curves should be considered qualitative rather than quantitative. Nonetheless, given that all the other conditions of cell preparation and drug application were the same for the different mice, it appears that the absence P2X1 subunits renders the P2X4 receptors less sensitive to ATP. Such an interpretation assumes that the macrophages under these conditions, express two separate populations of homomeric P2X1 and P2X4 receptors. But there is evidence from heterologous expression that P2X1 and P2X4 subunits can also form heteromeric channels (Nicke et al., 2005). However, the P2X1/4 heteromer that results from co-expression of P2X1 and P2X4 subunits can be activated by αβmeATP and has relatively slow kinetics (Nicke et al., 2005): we did not observe such a phenotype. An alternative explanation for the apparent insensitivity to ATP of cell from the P2X1−/− might be that receptors normally function in the membrane as part of a larger complex, and that this results in non-independent behaviour of the individual channel proteins, such as has been reported for homomeric P2X2 receptor channels (Ding and Sachs, 2002).
Acknowledgments
This work was supported by the Wellcome Trust and by Brain Research Center of the 21st Century Frontier Research Program (Grant M103KV010015-06K2201-01510) funded by the Ministry of Science and Technology, Republic of Korea. We thank Kyriaki Dossi for tissue culture, and genotyping.
Abbreviations
- αβmeATP
αβ-methyleneadenosine 5′-triphosphate
- IL-1β
interleukin-1β
- TNP-ATP
2′,3′-O-(2,4 6-trinitrophenyl)adenosine-5′-triphosphate
- LPS
lipopolysaccharide
- NF 449
4,4′,4″,4′″-[carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino))]tetrakis-benzene-1,3-disulphonic acid
- PPADS
pyridoxal-phosphate-6-azophenyl-2′4′-disulphonic acid tetrasodium
Conflict of interest
The authors state no conflict of interest.
References
- Bowler JW, Bailey RJ, North RA, Surprenant A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol. 2003;140:567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170:3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- Chen YW, Donnelly-Roberts DL, Namovic MT, Gintant GA, Cox BF, Jarvis MF, et al. Pharmacological characterization of P2X7 receptors in rat peritoneal cells. Inflamm Res. 2005;54:119–126. doi: 10.1007/s00011-004-1332-7. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Ojcius DM, Gorecki DC, Persechini PM, Biaggio RC, Mendes AN, et al. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. Evidence for non-independent gating of P2X2 receptor expressed in Xenopus oocytes. BMC Neurosci. 2002;3:17–28. doi: 10.1186/1471-2202-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschke D, Wust M, Hauschildt S, Nieber K. Pharmacological characterization of the P2X7 receptor on human macrophages using the patch-clamp technique. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:168–171. doi: 10.1007/s00210-001-0501-2. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- Gallin EK. Ion channels in leukocytes. Physiol Rev. 1991;71:775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- Ikeda M. Characterization of functional P2X1 receptors in mouse megakaryocytes. Thromb Res. 2007;119:343–353. doi: 10.1016/j.thromres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, et al. Functional characterization of the P2X4 receptor orthologues. Br J Pharmacol. 2000;129:388–394. doi: 10.1038/sj.bjp.0703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Banati R, Walz W. Electrophysiological behavior of microglia. Glia. 1993;7:93–101. doi: 10.1002/glia.440070115. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Spelta V, Sim J, North RA, Surprenant A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem. 2001;276:23262–23627. doi: 10.1074/jbc.M102253200. [DOI] [PubMed] [Google Scholar]
- Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem. 1999;274:36944–36951. doi: 10.1074/jbc.274.52.36944. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Owens GP, Hahn WE, Cohen JJ. Identification of mRNAs associated with programmed cell death in immature thymocytes. Molec Cell Biol. 1991;11:4177–4188. doi: 10.1128/mcb.11.8.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G. Desensitization masks nanomolar potency of ATP for the P2X1 receptor. J Biol Chem. 2004;279:6426–6433. doi: 10.1074/jbc.M306987200. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, et al. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter R, Barden JA, Wiley JS. Detection of P2X purinergic receptors on human B lymphocytes. Cell Tissue Res. 2001;304:231–236. doi: 10.1007/s004410100372. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Development and differentiation of macrophages and related cells: historical review and current concepts. J Clin Exp Hem. 2000;41:1–33. [Google Scholar]
- Townsend-Nicholson A, King BF, Wildman SS, Burnstock G. Molecular cloning, functional characterization and possible cooperativity between the murine P2X4 and P2X4a receptors. Brain Res Mol Brain Res. 1999;64:246–254. doi: 10.1016/s0169-328x(98)00328-3. [DOI] [PubMed] [Google Scholar]
- van Furth R.Production and migration of monocytes and kinetics of macrophages Mononuclear Phagocytes. Biology of monocytes and macrophages 1992Kluwer Academic Publishers: Dordrecht; 3–12.In: van Furth R (ed). [Google Scholar]
- Visentin S, Renzi M, Frank C, Greco A, Levi G. Two different ionotropic receptors are activated by ATP in rat microglia. J Physiol. 1999;519:723–736. doi: 10.1111/j.1469-7793.1999.0723n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;3:5–16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Seward EP, Buell GN, North RA. Domains of P2X receptors involved in desensitization. Proc Natl Acad Sci USA. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]