Abstract
The bacterial pathogen Pseudomonas viridiflava possesses two pathogenicity islands (PAIs) that share many gene homologs, but are structurally and phenotypically differentiated (T-PAI and S-PAI). These PAIs are paralogous, but only one is present in each isolate. While this dual presence/absence polymorphism has been shown to be maintained by balancing selection, little is known about the molecular evolution of individual genes on the PAIs. Here we investigate genetic variation of 12 PAI gene loci (7 on T-PAI and 5 on S-PAI) in 96 worldwide isolates of P. viridiflava. These genes include avirulence genes (hopPsyA and avrE), their putative chaperones (shcA and avrF), and genes encoding the type III outer proteins (hrpA, hrpZ, and hrpW). Average nucleotide diversities in these genes (π = 0.004–0.020) were close to those in the genetic background. Large numbers of recombination events were found within PAIs and a sign of positive selection was detected in avrE. These results suggest that the PAI genes are evolving relatively freely from each other on the PAIs, rather than as a single unit under balancing selection. Evolutionarily stable PAIs may be preferable in this species because preexisting genetic variation enables P. viridiflava to respond rapidly to natural selection.
GENE-for-gene interactions between plants and plant pathogens involve specific resistance (R) gene products in plants that are responsible for the recognition of elicitors encoded by avirulence (Avr) genes in pathogens (Flor 1971). Plant genomes possess many R gene loci (Arabidopsis Genome Initiative 2000); annotation of the Arabidopsis thaliana genomic sequence, in particular, identifies ∼150, most of which occur in clusters of 2–9 loci (Meyers et al. 2003). It is clear that selection has acted to diversify paralogous R gene family members in the Arabidopsis genome (Meyers et al. 2003). However, many R gene polymorphisms in A. thaliana have been shown to be subject to balancing or transient balancing selection, rather than directional selection (Stahl et al. 1999; Bergelson et al. 2001; Tian et al. 2002; Bakker et al. 2006; Shen et al. 2006), in contrast to expectations under the simplest arms race model (Dawkins and Krebs 1979). In fact, there is little evidence for direct R–Avr protein interaction (e.g., Dodds et al. 2006). These facts may be explained by the “guard hypothesis”, in which R gene products are assumed to recognize Avr gene products only indirectly via modifications of host proteins that are targets of the Avr gene products (Dangl and Jones 2001).
Much less is known about the molecular evolution of genes encoding bacterial effectors. Like Arabidopsis R genes, however, the Phyophthora infestans scr74 gene family of effectors may be subject to diversifying selection (Liu et al. 2005). Limited evidence of balancing selection acting upon virulence-related genes is found in Fusarium graminearum (Ward et al. 2002) and in Borrelia burgdorferi (Qiu et al. 2004). A comprehensive survey of Pseudomonas syringae effectors identified an additional seven gene families with domains subject to diversifying selection, although the majority of gene families were shaped by purifying selection (Rohmer et al. 2004). Rohmer et al. (2004) also showed that 9 of 24 effector genes were acquired via horizontal gene transfer (HGT), indicating that HGT is one of the important factors in bacterial effector gene evolution.
In a previous study, we identified two paralogous PAIs in P. viridiflava (Araki et al. 2006), a prevalent natural pathogen of A. thaliana (Jakob et al. 2002). Although these PAIs (T- and S-PAI, respectively) share 25 gene homologs, they are structurally distinct. The T-PAI is composed of three parts: a gene cluster encoding the type III protein-secretion apparatus (hrp/hrc gene cluster), the 5′-effector locus (exchangeable effector locus, EEL), and the 3′-effector locus (conserved effector locus, CEL). The S-PAI, on the other hand, has a single component hrp/hrc cluster with a 10 kb-long insertion. The two PAIs are integrated at different locations in the P. viridiflava genome, and both islands are segregating for alleles in which the entire island is deleted (Figure 1). Extensive surveys of P. viridiflava isolates from worldwide collections have identified only two combinations, each containing one of the two islands (i.e., [T-PAI, ∇S-PAI] and [∇T-PAI, S-PAI]). Several lines of evidence indicate that this dual presence/absence polymorphism has been maintained by balancing selection. Furthermore, the [T-PAI, ∇S-PAI] and [∇T-PAI, S-PAI] isolates exhibit virulence differences (Araki et al. 2006). [T-PAI, ∇S-PAI] isolates elicit a rapid defense response known as the hypersensitive response (HR) in A. thaliana significantly more slowly than [∇T-PAI, S-PAI] isolates, whereas they elicit an HR in tobacco significantly more quickly than the [∇T-PAI, S-PAI] isolates. This functional differentiation of PAIs is required for selection to maintain this unusual dual-haplotype configuration as a polymorphism. Why the entire PAI, rather than a particular effector gene on it, has been selected as a unit has been unclear and how individual genes on the PAI evolve remains unanswered.
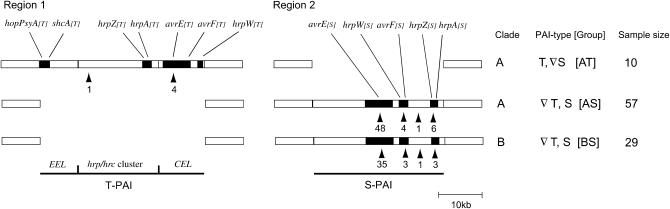
Figure 1.—
Schematics for locations of the PAI genes investigated in this study. T-PAI is located in region 1 (left) and S-PAI is located in region 2 (right). Physical distance of the two regions is unknown in P. viridiflava. [AT], [AS], and [BS] represent [clade, PAI-type] of the isolates. In all the samples, the presence of T-PAI in region 1 was perfectly associated with the absence of S-PAI in region 2, and vice versa. Arrowheads represent locations where at least one recombination event was detected by Hudson and Kaplan's Rm (1985), and the number below the arrowhead represents Rm found within or between loci.
To address these questions, we investigate molecular evolution in 12 PAI gene loci (7 on T-PAI and 5 on S-PAI) in P. viridiflava. These loci include five genes shared by the two PAIs (avrE, avrF, hrpA, hrpZ, and hrpW designated as avrE[S], avrF[S], hrpA[S], hrpZ[S], and hrpW[S] on S-PAI and avrE[T], avrF[T], hrpA[T], hrpZ[T], and hrpW[T] on T-PAI) as well as two T-PAI-specific genes (hopPsyA[T] and shcA[T]) (Figure 1). hopPsyA and shcA encode an Avr protein that elicits a HR on tobacco and its putative chaperone, respectively (Alfano and Collmer 1997; Van Dijk et al. 2002). avrE encodes a known Avr protein that elicits HR on tobacco and soybean plants (Lorang and Keen 1995). avrF is putatively an AvrE-specific chaperone (Bogdanove et al. 1998) and might be required for efficient delivery of AvrE (Ham et al. 2006). A potential role of AvrE as a suppressor of basal immunity and promotion of host cell death is reported in Debroy et al. (2004). hrpA, hrpZ, and hrpW encode outer proteins involved in the type-III secretion system (TTSS). hrpA encodes the pilus, which plays a key role in the secretion of Avr and other type-III effector proteins and is subject to natural selection in P. syringae (Jin and He 2001; Guttman et al. 2006). hrpZ and hrpW encode harpin proteins (Huang et al. 1995; Preston et al. 1995), which also elicit HR in the apoplast (in contrast to Avr proteins that elicit HR in plant cells; Wei et al. 1992; He et al. 1993). These genes, therefore, are likely to be involved in the Arabidopsis–P. viridiflava interaction, and hence subject to natural selection. Considering the range of putative functions of these gene products, however, the types and degrees of selective pressures on these genes might vary. Thus, one might expect that these PAI genes evolve in an independent fashion subject to different selective pressures (e.g., negative or positive selection). An alternative hypothesis is that the balancing selection on the presence/absence polymorphisms for entire PAIs overcomes the selective pressure on each PAI gene. In this case, one would expect that these genes evolve in concert.
We used 96 bacterial isolates collected mostly from A. thaliana leaves in worldwide populations, including the same isolates as previously investigated (Goss et al. 2005; Araki et al. 2006). P. viridiflava consists of two genetically differentiated clades, A and B (Goss et al. 2005). Both T- and S-PAI isolates are present in clade A, but only S-PAI isolates have been identified in clade B. We therefore analyzed genetic variation and molecular evolution of the 12 loci for each of the three groupings: 7 loci on [AT] (indicating isolates with the T-PAI in clade A) and 5 on [AS] (indicating isolates with the S-PAI in clade A) and [BS] (indicating isolates with the S-PAI in clade B) (Figure 1). Our results suggest that these loci have evolved relatively freely from other genes on the same PAI, despite the fact that they are located on genetic islands and that entire PAIs are under balancing selection.
MATERIALS AND METHODS
Samples:
Ninety-three isolates out of the 96 investigated here are described in Goss et al. (2005). Eighty-three of them were isolated from A. thaliana plants and the remaining 10 were isolated from other weedy plant species alongside A. thaliana. Three additional isolates included in this study—LU1.1a and LU18.1a (from Lund, Sweden) and KY12.1d (from Kyoto, Japan)—were isolated from A. thaliana plants. The PAI presence/absence genotype was identified by PCR (see Araki et al. 2006 for details). In this manner, 10 isolates were identified as [AT], 57 were identified as [AS] and 29 were identified as [BS].
Sequence data analyses and the tests of selective neutrality:
Sequences of the seven PAI genes were obtained by direct PCR and sequencing using the same conditions as those described in Araki et al. (2006). Primers for the PCR are listed in supplemental Table 1 at http://www.genetics.org/supplemental/. Primer annealing temperatures were between 55° and 60°. Sequences of hrpW[T] from three isolates (LU5.1a, LU9.1e, and PT220.1a) and hrpW[S] from four isolates (KNOX230.1a, ME751a, ME753a, and ME756a) were eliminated from the following analyses because we could obtain only partial sequences. Sequences were edited by Sequencher v.4.1.2 (Gene Codes, Ann Arbor, MI). After editing, sequences were aligned by CLUSTAL X (Thompson et al. 1997) with minor manual adjustments. Reference sequences for alignments were obtained from the GenBank online database as follows: P. viridiflava LP23.1a, PNA29.1a, and P. syringae pv. tomato (Pto) DC3000 for T-PAI and P. viridiflava ME3.1b, RMX23.1a, RMX3.1b, and P. cichorii 83-1 for S-PAI (Araki et al. 2006). Polymorphism and divergence surveys, selective neutrality tests, and a permutation test for genetic differentiation with 10,000 replicates were performed using DnaSP v4.00 (Rozas et al. 2003). Neighbor-joining (NJ) trees (Saitou and Nei 1987) were constructed by MEGA2.1 (Kumar et al. 2001) using Kimura's two-parameter model (Kimura 1980), the multilocus HKA test was conducted using the HKA program (available from http://lifesci.rutgers.edu/∼heylab/HeylabSoftware.htm#HKA) with 10,000 simulations, and the minimum-recombination number was estimated utilizing methods in Hudson and Kaplan (1985) and Myers and Griffiths (2003). Frequent recombination was further tested by a runs test using GENECONV v. 1.81 (based on a global permutation test after multiple-comparison correction; see Sawyer 1989).
RESULTS
Physical organization and structural evolution:
Figure 1 depicts the physical location of the 12 loci on the two paralogous PAIs (T-PAI in chromosomal region 1 and S-PAI in chromosomal region 2). T- and S-PAI genotypes were both represented among the 67 clade A isolates but only S-PAI genotypes were present in the 29 clade B isolates. Thus, our 96 samples of P. viridiflava consist of 10 [AT], 57 [AS], and 29 [BS] isolates. An Avr gene homolog, hopPsyA[T] and its putative chaperone, shcA[T], were located in the EEL region in the T-PAI, but is not present in the S-PAI. The avrE[S], avrF[S], and hrpW[S] were located in the 10-kb insertion in both [AS] and [BS], whereas homologs of these genes (avrE[T], avrF[T], and hrpW[T]) were found in the CEL region in [AT] isolates. Both hrpA and hrpZ occupied adjacent locations in the hrp/hrc cluster in both T-PAI (hrpA[T] and hrpZ[T]) and S-PAI (hrpA[S] and hrpZ[S]).
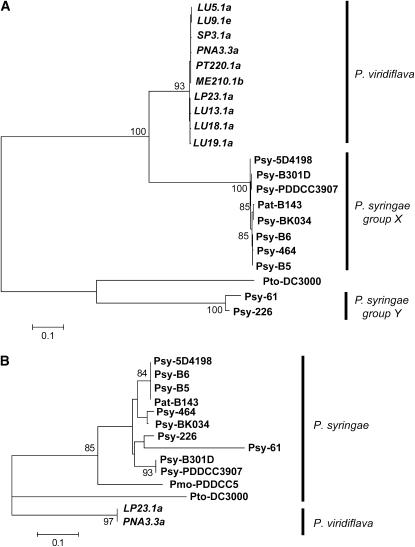
In the EEL region on the T-PAI, hopPsyA[T] and shcA[T] were located adjacent to one another and shared the same orientation in all 10 [AT] isolates. The consistent gene composition and gene orientation of these genes among P. viridiflava isolates contrasts to those in P. syringae. Among P. syringae pathovars, the EEL region is hypervariable both in gene composition and in gene orientation, which suggests frequent HGTs and genetic exchanges (Alfano et al. 2000; Charity et al. 2003; Deng et al. 2003). Indeed, phylogenetic analysis of hopPsyA and shcA among P. viridiflava and P. syringae isolates revealed that the genes in P. syringae isolates are split into two distantly related clades (groups X and Y in Figure 2A). The nucleotide sequences from P. viridiflava cluster together and are closely related to the P. syringae group X. This pattern was confirmed using several phylogenetic methods including maximum parsimony and the unweighted pair group method with arithmetic mean (UPGMA) (data not shown). Interestingly, all gene homologs in P. syringae group X share the same gene orientation as those in P. viridiflava, whereas those in P. syringae group Y have the opposite gene orientation. These results are consistent with the HGT of these two members of the EEL; horizontal transfer of the EEL region is further supported by the presence of tRNALeu in its 5′ end and its low G + C% in Pseudomonas (Charity et al. 2003; Araki et al. 2006). The data do not allow us to ascertain the direction or source of the HGT event, however. In the hrp/hrc cluster, on the other hand, no sign of HGT was found. Even the genealogy of partial sequences (175 bp) in hrpK[T], a gene in the hrp/hrc cluster next to hopPsyA[T] and shcA[T] in P. viridiflava, was consistent with the phylogeny of these species (Figure 2B). This result suggests that the hrp/hrc cluster is not included in the potential HGT of the EEL region, confirming previous reports in P. syringae (Sawada et al. 1999; Alfano et al. 2000; Charity et al. 2003).
Figure 2.—
Genealogical relationships of hopPsyA and shcA (A) and a part of hrpK (B). The neighbor-joining trees were constructed on the basis of the nucleotide variations in the third codon position in the combined coding sequences of hopPsyA + schA (1704 bp) and the partial sequences of hrpK (175 bp). The sequences in P. syringae were obtained from either Charity et al. (2003) or Deng et al. (2003). Bootstrap probabilities (%) with 1000 replicates, which were >80%, were represented on or below the major branch. The partial hrpK sequences in P. viridiflava were only available in LP23.1a and PNA3.3a (Araki et al. 2006).
The hrp/hrc gene cluster is composed of genes encoding the type-III protein-secretion apparatus. In the hrp/hrc gene cluster, hrpZ and hrpW encode harpin proteins, defined as glycine-rich, cysteine-lacking proteins that are secreted by the TTSS and that possess heat-stable HR elicitor activity (Wei et al. 1992; Huang et al. 1995; Charkowski et al. 1998). HrpZ in P. viridiflava shares these features of amino acid sequences, containing no cystein-coding codons and 42–46 out of 314–337 glycine-coding codons. HrpW in P. syringae is composed of two domains: the harpin domain in the 5′ half and the pectate lyase domain in the 3′ half (Charkowski et al. 1998). This structure was also found in P. viridiflava, and no cysteine-coding codon was found in hrpW in this species. However, the harpin domain is quite variable among groups in P. viridiflava (average π within species is 0.27 and 0.11 for synonymous and nonsynonymous sites, respectively), and the number of glycine-coding codons varies substantially even among isolates in the same group (supplemental Figure 1 at http://www.genetics.org/supplemental/). The average number (and standard deviation, SD) of glycine-coding codons in the harpin domain is 37.9 (SD = 0.1) for [AT], 116.1 (SD = 6.7) for [AS], and 115.2 (SD = 7.7) for [BS]. This compares to 40 glycine-coding codons in the harpin domain of Pto DC3000 and 119 glycine-coding codons in the harpin domain of P. cichorii 83-1. The pectate lyase domain in hrpW of P. viridiflava, on the other hand, is less polymorphic and amino acid sequence is well conserved both between species and between T-PAI and S-PAI in this domain (average π within species is 0.21 and 0.04 for synonymous and nonsynonymous sites, respectively). While the harpin domain in HrpW is known to be under positive selection in P. syringae (Rohmer et al. 2004), the functional and evolutionary significance of variable numbers of glycine-coding codons in this domain needs to be further addressed in P. viridiflava.
Level of polymorphism within lineages:
Synonymous nucleotide diversity (πsyn, Nei 1987) was estimated for each gene within each of the three PAI-clade groups ([AT], [AS], and [BS], Table 1). There are three notable observations in Table 1:
πsyn varies substantially among loci and among groups. This result suggests the possibility of different evolutionary histories among the PAI genes.
hrpA shows almost no genetic variation within each group. No variation was found in hrpA[T] among [AT] and in hrpA[S] among [BS] isolates. hrpA[S] among [AS] was nearly invariant.
No clear difference in the levels of genetic variation was found between T- and S-PAI and between the PAI genes and their genetic background.
TABLE 1.
Genetic variations of the PAI genes
| [AT]
|
[AS]
|
[BS]: | |||||
|---|---|---|---|---|---|---|---|
| Gene | Size (bp) | n | π (πsyn)a | n | π (πsyn)a | n | π (πsyn)a |
| shcA[T] | 378 | 10 | 0.0025 (0.0106) | 0 | — | 0 | — |
| hopPsyA[T] | 1146 | 10 | 0.0010 (0.0033) | 0 | — | 0 | — |
| avrE ([T] or [S]) | 5165 | 10 | 0.0041 (0.0101) | 57 | 0.0233 (0.0714) | 29 | 0.0130 (0.0425) |
| avrF ([T] or [S]) | 395 | 10 | 0.0135 (0.0508) | 57 | 0.0120 (0.0434) | 29 | 0.0079 (0.0306) |
| hrpA ([T] or [S]) | 217 | 10 | 0.0000 (0.0000) | 57 | 0.0013 (0.0054) | 29 | 0.0000 (0.0000) |
| hrpZ ([T] or [S]) | 952 | 10 | 0.0005 (0.0020) | 57 | 0.0104 (0.0373) | 29 | 0.0048 (0.0195) |
| hrpW ([T] or [S]) | 1474 | 7 | 0.0022 (0.0040) | 54 | 0.0185 (0.0559) | 28 | 0.0229 (0.0739) |
| Averageb | — | — | 0.0036 (0.0096) | — | 0.0199 (0.0616) | — | 0.0133 (0.0441) |
| E[θ]c | — | — | 0.0241 (0.0646) | — | 0.0233 (0.0725) | — | — |
Average nucleotide diversity (Nei 1987). Numbers in parentheses represent the average nucleotide diversity of synonymous sites.
Average over the five PAI genes shared between T- and S-PAI (avrE, avrF, hrpA, hrpZ, and hrpW).
Estimate of θ = 2Nμ, where N is an effective population size of clade A and μ is the mutation rate, was calculated from the average nucleotide diversities of [AT] and [AS] on the basis of Innan and Tajima (1997), assuming random mating and 10/67 and 57/67 of [AT] and [AS] frequencies in the clade A population, respectively.
The average nucleotide diversity observed in the PAI genes is low in [AT] (π = 0.004), which is only 18 and 27% of that in [AS] and [BS] (Table 1). However, the low level of genetic variation in [AT] was expected a priori because [AT] isolates occur at ∼10% frequency in the worldwide sample. Under neutral equilibrium model assumptions, estimates of the neutral parameter θ = 2Nμ (N is an effective population size and μ is the mutation rate) were very close for [AT] (0.024) and [AS] (0.023) after adjusting for the PAI frequency difference in clade A (Innan and Tajima 1997) (Table 1). Those values of θ and that in [BS] (0.013, S-PAI is fixed in clade B) were also very similar to the levels of polymorphism found in the background loci for these isolates (π = 0.022 in clade A and 0.009 in clade B; Goss et al. 2005).
Genetic divergence:
Genetic divergence of the PAI gene homologs among groups in P. viridiflava and between species (P. syrinage for T-PAI and P. cichorii for S-PAI, Araki et al. 2006) is shown in Table 2. T-PAI and S-PAI gene homologs are extensively diverged (Dxy = 0.624–1.860, Nei 1987), confirming that the most recent common ancestor between T- and S-PAI predates the split of P. syringae and P. cichorii from their most recent common ancestors with P. viridflava (Figure 3) (see also Araki et al. 2006).
TABLE 2.
Genetic diversities of the PAI genes between lineages (Dxy)
| shcA | hopPsyA | avrE | avrF | hrpA | hrpZ | hrpW | |
|---|---|---|---|---|---|---|---|
| Within P. viridiflava (orthologous) | |||||||
| [AS] vs. [BS] | — | — | 0.086 (0.284) | 0.079 (0.326) | 0.014 (0.064) | 0.051 (0.217) | 0.093 (0.367) |
| Within P. viridiflava (paralogous) | |||||||
| [AT] vs. [AS] | — | — | 0.739 (1.545) | 0.659 (N.A.) | 1.744 (1.833) | 1.046 (2.544) | 0.811 (1.592) |
| [AT] vs. [BS] | — | — | 0.735 (1.462) | 0.624 (3.730) | 1.860 (2.713) | 1.044 (2.280) | 0.822 (1.727) |
| Between species (orthologous) | |||||||
| [AT] vs. Pto DC3000 | 0.498 (1.565) | 0.801 (2.915) | 0.431 (1.657) | 0.338 (2.941) | 0.929 (1.113) | 0.523 (1.654) | 0.491 (1.667) |
| [AS] vs. Pcic 83-1 | — | — | 0.568 (1.431) | 0.282 (0.917) | 0.140 (0.426) | 0.244 (0.862) | 0.309 (1.037) |
| [BS] vs. Pcic 83-1 | — | — | 0.569 (1.381) | 0.292 (0.970) | 0.134 (0.408) | 0.260 (1.052) | 0.299 (0.997) |
Genetic diversities (Dxy) were calculated on the basis of Nei (1987) with Jukes and Cantor (1969) correction. Values within parenthesis represent Dxy in synonymous sites. Pto DC3000 and Pcic 83-1 represent P. syringae DC3000 and P. cichorii 83-1, which are outgroups with the T-PAI and S-PAI homolog, respectively (Araki et al. 2006). Dxy in the synonymous sites of avrF could not be obtained because this value was beyond the level of the Jukes and Cantor (1969) correction.
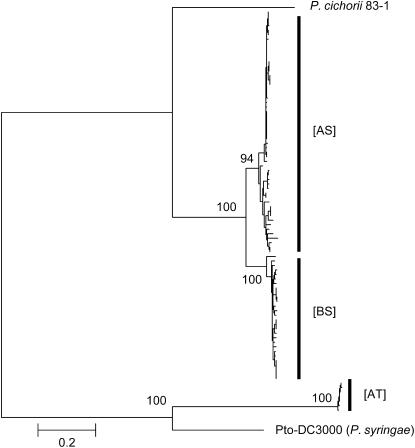
Figure 3.—
Genealogical relationships of five shared genes between T-PAI and S-PAI. The neighbor-joining trees were constructed on the basis of the nucleotide variations in the third codon position in the combined coding sequences of the five genes (aveE, avrF, hrpA, hrpZ, and hrpW, 6720 bp comparable in total) after the Jukes–Cantor correction (Kumar et al. 2001). Bootstrap probabilities (percentage) with 1000 replicates were represented on or below the major branches.
Comparisons between [AS] and [BS] reveal a moderate level of genetic divergence (Dxy = 0.014–0.086), similar to that of the background loci in these isolates (Dxy = 0.037–0.087, Goss et al. 2005). Levels of genetic divergence between [AS] and P. cichorii were very similar to those between [BS] and P. cichorii (Table 2), indicating similar rates of divergence of the two P. viridiflava clades. These patterns of divergence are consistent with a model of vertical phylogenetic transmission of the S-PAI loci within species.
Comparisons between [AT] isolates in P. viridiflava and Pto DC3000, on the other hand, revealed that synonymous divergence of the orthologous PAI genes (Dxy = 1.1–2.9, average 1.6) is consistently greater than for the background loci (Dxy = 0.49–0.73, average 0.56, Goss et al. 2005). While the cause of the large divergence is not clear, the genealogy of the third codon sequences from the five genes shared between T- and S-PAI (Figure 3) indicates a higher evolutionary rate of these genes in [AT] relative to that in Pto DC3000. According to the genetic distance between these lineages (Figure 3), the evolutionary rates of the PAI genes among the [AT] lineages are on average 48.3% higher than those in Pto DC3000 after the split of these lineages. Although Tajima's relative-rate test (Tajima 1993) did not detect a statistical significance of the difference in the evolutionary rates between them (P = 0.36–0.47, P. cichorii as an outgroup), this result may be due to lack of statistical power because P. cichorii was highly divergent from these lineages. Thus, our data are basically consistent with the increased evolutionary rate of the PAI genes among [AT] lineages rather than in P. syringae.
Recombination within each PAI:
Within each PAI, patterns of genetic variation provided clear signs of recombination, despite the tight physical linkage of the PAI gene loci. We estimated the minimum number of recombination events (Rm) between sequences within [AT], [AS], and [BS] using the very conservative method of Hudson and Kaplan (1985) (Table 3 and Figure 1). The estimated Rm in S-PAI is very large (Rm[AS] = 59 and Rm[BS] = 42), whereas that in T-PAI is moderate (Rm[AT] = 5). The small number of recombination events detected in [AT] must be, at least in part, a consequence of small sample size (10 isolates) and low genetic variation in [AT] (Table 1). Regardless, these are impressively large numbers, especially given the fact that this conservative method identifies only a small fraction of the actual recombination events in the history of a sample (Hudson and Kaplan 1985). The majority of the recombination events was detected within avrE in both PAIs, the largest gene locus in each PAI (>5 kb, Figure 1). Qualitatively similar results were also obtained by different methods (Sawyer 1989; Myers and Griffiths 2003) (Table 3). These results are consistent with those of Goss et al. (2005), in which a high rate of recombination is reported among genomewide, background loci in this species. The large numbers of recombination events within the small genomic islands (<30 kb each) found in this study provide further evidence of the high recombination rate in this species.
TABLE 3.
Detected number of recombination events within each group
| Method | [AT] | [AS] | [BS] |
|---|---|---|---|
| Hudson and Kaplan's (1985)Rm | 5 | 59 | 42 |
| Myers and Griffiths's (2003)Rm | 5 | 86 | 82 |
| Sawyer's (1989) run test | 10 | 166 | 35 |
Between T-PAI and S-PAI, on the other hand, the large genetic divergence (Table 2, Figure 3) suggests that paralogous recombination (i.e., gene conversion) is inefficient in exchanging the nucleotide sequences between the two.
Tests of selective neutrality:
We investigated the compatibility of polymorphism and divergence patterns with selective neutrality by applying statistical tests on the basis of (1) allele frequencies (Tajima 1989; Fu and Li 1993), (2) polymorphism level differences among loci (HKA test) (Hudson et al. 1987), (3) the distribution of synonymous and nonsynonymous polymorphism and divergence (MK test) (McDonald and Kreitman 1991), and (4) the ratio of nonysynonymous:synonymous divergence (Ka/Ks).
Strongly negative (or positive) values of Tajima's D and Fu and Li's D* and F* statistics at individual loci can be interpreted as evidence for positive directional (or balancing) selection. No significant deviation from selective neutrality was detected by these tests for any of the 12 loci (data not shown).
The HKA test is a conservative test of neutrality that asks whether polymorphism levels across loci are compatible with neutral evolution, taking into account possible functional constraint or mutation-rate differences between the loci. This test, and the MK test below, is best applied using divergence data between species (or clades) that are closely related to avoid complications due to multiple substitutions at individual nucleotide sites. In our data, generally large genetic diversities between species and between T- and S-PAI (Dxy > 1, Table 2) suggested that these data are not suitable for this neutrality test. Therefore, we restricted application of the HKA test to the comparison of polymorphism levels among the five loci in [AS] and [BS]. For these genes, neither the multilocus HKA test (P = 0.86) nor the pairwise HKA test (P = 0.22 [hrpA[S]-hrpW[S]]–0.94 [hrpZ[S]-avrE[S]]) detected departure from selective neutrality.
The ratio of synonymous and replacement substitutions between species (or clades) can be compared with the same ratio for polymorphisms within a species (or a clade) as a test for adaptive protein evolution (McDonald and Kreitman 1991). As above, we restricted the application of this test to protein evolution within and between [AS] and [BS] PAI genes (Table 4). Of the four genes that could be tested (avrE[S], avrF[S], hrpZ[S], hrpW[S]), the MK test detected a significant departure from selective neutrality (in the direction of adaptive protein evolution) for avrE[S] (P = 0.035). The avrF[S] has a shorter coding region and many fewer substitutions than avrE[S] and showed no sign of positive selection between [AS] and [BS] (P = 1.0). Note that the hrpA[S] coding region is very short (68 codons) and is nearly invariant both within and between [AS] and [BS] clades. Thus we could not carry out a MK test for this gene.
TABLE 4.
Distribution of substitutions between AS and BS
| Substitutions
|
||||||
|---|---|---|---|---|---|---|
| Locus | No. of codon analyzed | Fix-syn. | Fix-rep. | Pol-syn. | Pol-rep. | P |
| avrE[S] | 1715 | 130 | 485 | 72 | 186 | 0.035 |
| avrF[S] | 132 | 10 | 35 | 2 | 6 | 1.000 |
| hrpA[S] | 68 | 2 | 2 | 0 | 0 | — |
| hrpZ[S] | 314 | 23 | 58 | 3 | 10 | 0.755 |
| hrpW[S] | 471 | 36 | 166 | 6 | 54 | 0.166 |
The P-value was calculated from the MK test (McDonald and Kreitman 1991) using Fisher's exact test. None of them was statistically significant after Bonferroni correction for multiple comparisons. Fix-syn., Fix-rep., Pol-syn., and Pol-rep.: fixed synonymous, fixed replacement, polymorphic synonymous, and polymorphic replacement substitutions, respectively.
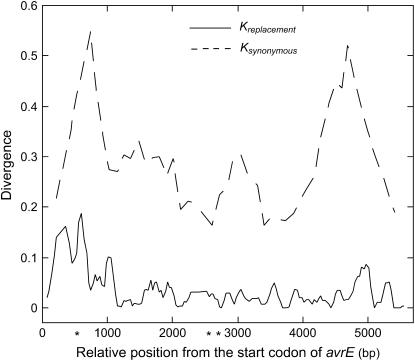
To further investigate the distribution of replacement substitutions between [AS] and [BS] in avrE[S], we performed a sliding window analysis (Figure 4). A high level of genetic divergence between [AS] and [BS] was found in the first 1 kb of avrE[S]. Similar distributions of replacement substitutions were found among other comparisons of avrE (between species and between T-PAI and S-PAI) and even between [BS] isolates from different geographic regions (data not shown). A less conserved amino acid sequence of the first half of avrE is reported between P. syringae and Erwinia amylovora (Bogdanove et al. 1998), indicating that the highly variable N-terminal of AvrE protein is common in plant pathogens. Whether the elevated genetic divergence is due to positive selection or an absence of selective constraint in this region is not clear, but Fu and Li's D* and F* showed significant deviation from the selective neutrality in a window of 392–503 bp (from the start codon, D* = −2.35, F* = −2.46, P < 0.05) when we examined avrE[S] in [BS] with an outgroup from [AS]. This window was one of three windows that showed significant departure from neutrality both by D* and F* (all negative, Figure 4) and no significant departure was detected by these statistics when we examined avrE[S] in [AS] with an outgroup from [BS]. These results indicate a possibility of positive selection on the N-terminal of AvrE.
Figure 4.—
Window plot analysis of nucleotide divergence between [AS] and [BS] in avrE. The genetic divergences in replacement sites (solid line) and in synonymous sites (dashed line) are plotted with relative position of the start position of the coding region in avrE. The window size and step size were 100 and 25 bp, respectively. An asterisk represents a midpoint of the windows that showed statistical significance (P < 0.05) by both Fu and Li's D* and F* in [BS] with a sequence from [AS] as an outgroup. D* = −2.35 and F* = −2.46 in a window of 392–503 (bp from the start codon), D* = −2.35 and F* = −2.46 in a window of 2493–2592, and D* = −2.41 and F* = −2.77 in a window of 2718–2817.
Although we could not carry out a MK test for hrpA, this gene is highly differentiated between [AT] and Pto DC3000, and between T- and S-PAI (Table 2). The Ka/Ks ratio of hrpA in these comparisons is also moderately high (see below).
The leucine-rich repeat domain of R proteins in A. thaliana and other plant species, which is generally believed to encode protein-recognition sites, can evolve rapidly, with nonsynonymous:synonymous substitution ratios (Ka/Ks) exceeding one (reviewed in Bergelson et al. 2001). To examine whether Avr proteins have similarly fast rates of evolution, we calculated the Ka/Ks ratios for the seven genes (Table 5). None of them produced Ka/Ks > 1. The Ka/Ks ratio in hrpA was moderately high between [AT] and Pto DC3000 (Ka/Ks = 0.79) and between T-PAI and S-PAI (Ka/Ks = 0.64–0.94). Although there remains a possibility that only a part of the coding sequences are subject to the positive selection and hence Ka/Ks > 1, window plot analyses for the Ka/Ks ratio generally did not detect regional Ka/Ks > 1 in the seven genes. A single exception was 600 bp at the 5′ end of avrE, which showed high Ka/Ks between species (e.g., between [AS] and P. cichorii 83-1, Ka/Ks = 0.3–4.2, average 1.2).
TABLE 5.
Ka/Ks ratio among PAI genes
| shcA | hopPsyA | avrE | avrF | hrpA | hrpZ | hrpW | |
|---|---|---|---|---|---|---|---|
| Within P. viridiflava (orthologous) | |||||||
| [AS] vs. [BS] | — | — | 0.115 | 0.041 | 0.000 | 0.034 | 0.052 |
| Within P. viridiflava (paralogous) | |||||||
| [AT] vs. [AS] | — | — | 0.387 | — | 0.938 | 0.345 | 0.419 |
| [AT] vs. [BS] | — | — | 0.411 | 0.112 | 0.635 | 0.389 | 0.386 |
| Between species (orthologous) | |||||||
| [AT] vs. Pto DC3000 | 0.257 | 0.208 | 0.157 | 0.047 | 0.792 | 0.219 | 0.198 |
| [AS] vs. Pcic 83-1 | — | — | 0.295 | 0.168 | 0.165 | 0.148 | 0.161 |
| [BS] vs. Pcic 83-1 | — | — | 0.309 | 0.163 | 0.164 | 0.121 | 0.156 |
Missing data are either by no sample (no shcA and hopPsyA in [AS] and [BS]) or failure of Jukes and Cantor (1969) correction (avrF between [AT] and [AS]).
DISCUSSION
The main goal of this study was to explore how genes involved in pathogencity have evolved on two PAIs that have already been determined to be under balancing selection in P. viridiflava. Three principle results emerged from our analyses: (1) the levels of genetic variation varied among the PAI genes, but were in a range of genetic variation observed in the background, (2) substantial numbers of recombination events were detected within each PAI, and (3) there was no statistical evidence for positive selection in most of the PAI genes, with the exception of avrE. Our results, considered jointly with those previously reported in Araki et al. (2006), suggest that two different levels of selection need to be considered. At the PAI level as a whole, two distinct systems of presumably coevolved loci (T- and S-PAI) are maintained by balancing selection, most likely because of their virulence differences and host specificities (Araki et al. 2006). On the other hand, the extensive reshuffling of polymorphism within each PAI by homologous recombination indicates that the entire PAI is not evolving as a single linkage block in P. viridiflava. Rather, there appears to be considerable independence in the genealogical histories of individual loci and ample opportunity for simultaneous adaptive evolution of constituent PAI genes. That the components of the enteroocyte effacement island in Escherichia coli are, at best, weakly coupled in their evolution, has similarly been reported (Castillo et al. 2005).
Functional significance of the PAI genes:
The functional significance of the genes investigated is largely unknown in P. viridiflava. However, several lines of evidence indicate that the PAI genes investigated here are functional. First, no frameshift or null mutations were observed in any of the coding sequences of these genes in any of the sample of 96 isolates. Second, nonsynonymous rates of evolution are uniformly lower than the corresponding synonymous rates across all within- and between-species comparisons, and are therefore entirely compatible with selective constraints on amino acid substitutions. Together, the lack of frameshifts and the relatively low nonsynonymous-substitution rates are solid indicators of protein functionality.
In addition, a TTSS-specific promoter domain, hrp box, was found to be conserved among the PAIs. The hrp box was originally defined as GGAACC-N15 or 16-CCACNNA in P. syrinage (Xiao and Hutcheson 1994). The hrp box has been found in the 5′-flanking regions of many TTSS-related genes in a broad range of plant pathogens, allowing some redundancies (Arnold et al. 2001; Rantakari et al. 2001). In P. viridiflava, we identified 12 hrp boxes in the T-PAI and 9 in the S-PAI (supplemental Table 2 at http://www.genetics.org/supplemental/). Hrp-box sequences were identified for 7 of the 12 loci (avrE[T], avrE[S], avrF[S], hrpA[T], hrpA[S], hrpW[T], and hrpW[S]) in all the isolates investigated in P. vidiflava. For the other 5 loci (avrF[T], hrpZ[T], hrpZ[S], shcA[T], and hopPsyA[T]), we did not find hrp boxes, but these genes may belong to operons in which the promoters are located some distance from individual genes (as reported in P. syringae; Alfano et al. 2000). The identified hrp boxes (supplemental Table 2 at http://www.genetics.org/supplemental/) show that the hrp box is relatively well conserved among genes and lineages, in contrast to the high levels of genetic divergence in the coding regions between the paralogous PAIs. This selective constraint on promoter sequences is another good indicator of functional expression of these genes.
Finally, we have shown that both T-PAI isolates and S-PAI isolates are capable of producing pilli when grown on inducing medium and induce the salicylic-acid-mediated host defense pathway when grown in Arabidopsis (Jakob et al. 2007). While not definitive proof, this does suggest that the type-III secretion system in these isolates is operational in this species.
Evolution of PAI genes in P. viridiflava:
We found a potential HGT of hopPsyA and shcA between P. syringae and P. viridiflava. We cannot determine a specific route of the HGT by this genealogy alone because there are many possible ways to end up with the observed genealogy (Figure 2A). This is especially true if we take possible HGTs from uninvestigated species into consideration. If we assume a single HGT between P. viridiflava and P. syringae, however, Figure 2A suggests that the HGT did not occur very recently because the level of genetic divergence in these genes is high even between P. viridiflava and P. syringae group X (D[P. viridiflava-group X] = 0.227). The extensive variation in the gene repertoire in the EEL region in P. syringae (Alfano et al. 2000; Charity et al. 2003; Deng et al. 2003), which contrasts the uniform EEL gene composition in P. viridiflava, suggests that the HGTs are more likely to occur in P. syringae rather than in P. viridiflava. It is still possible that the [AT] isolates acquired the observed EEL region relatively recently from an unknown species, but the moderate levels of genetic variation in hopPsyA and shcA comparable to those in the other genes among [AT] (Table 1) suggest the evolutionary stability of hopPsyA and shcA in P. viridiflava. More importantly, the genealogy of hrpK (Figure 2B) suggests no influence of HGT on the hrp/hrc cluster. Thus, the extent of a HGT in P. viridiflava, if any, must be limited to the EEL region.
We found a sign of positive selection in avrE, which is one of two Avr genes investigated in this study. One possible cause of selection on this gene is its virulence function, suggested by Venisse et al. (2003) and Debroy et al. (2004). According to Debroy et al. (2004), avrE in P. syringae functions as a suppressor of salicylic-acid-mediated basal defense, and amino acid changes in AvrE might affect this function. Our window plot analysis indicates the N-terminal region of AvrE is a good candidate of the target of natural selection.
hrpA[T] and hrpA[S] were nearly invariant within each locus but highly divergent between these paralogous loci (Tables 1 and 2). Within each locus, the low levels of polymorphism in hrpA indicate strong selective or mutational constraints. Strong selective constraints for conserving the protein structure of HrpA might be reasonable, considering that hrpA encodes a pilus that functions as a conduit for Avr protein delivery (Jin and He 2001). However, hrpA in P. syringae is reported to be under diversifying selection (Guttman et al. 2006), suggesting that modifications in protein structure of HrpA can be adaptive in Pseudomonas. Indeed, unlike within each locus, hrpA is highly divergent between hrpA[T] and hrpA[S] and between species (Table 2). While our data did not allow us to effectively test the selective neutrality of hrpA, this pattern of genetic variation in hrpA (nearly monomorphic within each locus and highly divergent between loci) may be caused by positive selection for functional differentiation of HrpA between loci, followed by negative selection for conserving HrpA within each locus in P. viridiflava.
Evolutionary significance of phylogenetically stable PAIs:
Comparable levels of polymorphism in the PAI genes and the extensive shuffling of variation among orthologous genes is a hallmark of vertical gene transmission. Horizontal transmission of foreign DNA into the PAI would introduce linked mutations on the allele in which the foreign sequence landed, but such an event would produce only one new haplotype. This would not be counted as a recombination event in the Hudson and Kaplan (1985) method, which requires the presence of all four possible combinations of two segregating sites (four haplotypes) to identify a recombination event. Thus, the results reported here are very consistent with the vertical gene transmission of the PAI genes in this species, as was reported in Araki et al. (2006).
While there is sound evidence that the evolution of pathogenesis involves the acquisition of virulence-related genes (including entire PAIs) via HGT (Reid et al. 2000; Bukhalid et al. 2002), some studies reported that PAIs have been retained within pathogen species over evolutionary time (e.g., Escobar-Paramo et al. 2003; Gressmann et al. 2005; Rohmer et al. 2004; Nallapareddy et al. 2005). We do not know why some PAIs are evolutionary stable whereas others are not, but our results provide one possible explanation: Unlike a PAI acquired via recent HGT, an evolutionary stable PAI can work as a large reservoir of preexisting genetic variation in this highly polymorphic species. It guarantees an immediate response to selection because the population does not have to wait for the next occurrence of a potentially adaptive mutation. Thus, the stable and polymorphic PAI may be favorable when rapid adaptive response is required. In addition, large population size (evidenced by the large standing crop of single nucleotide polymorphism) also increases the chance occurrence of a novel adaptive mutation in the population.
Acknowledgments
We thank D. Begun and two anonymous reviewers for helpful suggestions. This work was supported by NIH grant GM-62504 to J.B. and M.K. and by a fellowship from the Japan Society for the Promotion of Science to H.A.
References
- Alfano, J. R., and A. Collmer, 1997. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179: 5655–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano, J. R., A. O. Charkowski, W. L. Deng, J. L. Badel, T. Petnicki-Ocwieja et al., 2000. The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure composed of a cluster of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in plants. Proc. Natl. Acad. Sci. USA 97: 4856–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative, 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815. [DOI] [PubMed] [Google Scholar]
- Araki, H., D. Tian, E. M. Goss, K. Jakob, S. S. Halldorsdottir et al., 2006. Presence/absence polymorphism for alternative pathogenicity islands in Pseudomonas viridiflava, a pathogen of Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 5887–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, D. L., R. W. Jackson, A. J. Fillingham, S. C. Goss, J. D. Taylor et al., 2001. Highly conserved sequences flank avirulence genes: isolation of novel avirulence genes from Pseudomonas syringae pv. pisi. Microbiology 147: 1171–1182. [DOI] [PubMed] [Google Scholar]
- Bakker, E. G., C. Toomajian, M. Kreitman and J. Bergelson, 2006. A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 18: 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson, J., M. Kreitman, E. A. Stahl and D. Tian, 2001. Evolutionary dynamics of plant R-genes. Science 292: 2281–2285. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A. J., J. F. Kim, Z. Wei, P. Kolchinsky, A. O. Charkowski et al., 1998. Homology and functional similarity of an hrp-linked pathogenicity locus, dspEF, of Erwinia amylovora and the avirulence locus avrE of Pseudomonas syringae pathovar tomato. Proc. Natl. Acad. Sci. USA 95: 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhalid, R. A., T. Takeuchi, D. Labeda and R. Loria, 2002. Horizontal transfer of the plant virulence gene, nec1, and flanking sequences among genetically distinct Streptomyces strains in the diastatochromogenes cluster. Appl. Environ. Microbiol. 68: 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo, A., L. Equiarte and V. Souza, 2005. A genomic population genetics analysis of the pathogenic enterocyte effacement island in Escherichia coli the search for the unit of selection. Proc. Natl. Acad. Sci. USA 102: 1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charity, J. C., K. Pak, C. F. Delwiche and S. W. Hutcheson, 2003. Novel exchangeable effector loci associated with the Pseudomonas syringae hrp pathogenicity island: evidence for integron-like assembly from transposed gene cassettes. Mol. Plant-Microbe Interact. 16: 495–507. [DOI] [PubMed] [Google Scholar]
- Charkowski, A. O., J. R. Alfano, G. Preston, J. Yuan, S. Y. He et al., 1998. The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 180: 5211–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J. L., and J. D. Jones, 2001. Plant pathogens and integrated defense responses to infection. Nature 411: 826–833. [DOI] [PubMed] [Google Scholar]
- Dawkins, R., and J. R. Krebs, 1979. Arms race between and within species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 205: 489–511. [DOI] [PubMed] [Google Scholar]
- Debroy, S., R. Thilmony, Y. B. Kwack, K. Nomura and S. Y. He, 2004. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. USA 101: 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. L., A. H. Rehm, A. O. Charkowski, C. M. Rojas and A. Collmer, 2003. Pseudomonas syringae exchangeable effector loci: sequence diversity in representative pathovars and virulence function in P. syringae pv. syringae B728a. J. Bacteriol. 185: 2592–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P. N., G. J. Lawrence, A. M. Catanzariti, T. Teh, C. I. Wang et al., 2006. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA 103: 8888–8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Paramo, P., C. Giudicelli, C. Parsot and E. Denamur, 2003. The evolutionary history of shigella and enteroinvasive Escherichia coli revisited. J. Mol. Evol. 57: 140–148. [DOI] [PubMed] [Google Scholar]
- Flor, H. H., 1971. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9: 275–296. [Google Scholar]
- Fu, Y.-X., and W.-H. Li, 1993. Statistical tests of neutrality of mutations. Genetics 133: 693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss, E. M., M. E. Kreitman and J. Bergelson, 2005. Genetic diversity, recombination, and cryptic clades in Pseudomonas viridiflava infecting natural populations of Arabidopsis thaliana. Genetics 169: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressmann, H., B. Linz, R. Ghai, K. P. Pleissner, R. Schlapbach et al., 2005. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 1: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman, D. S., S. J. Gropp, R. L. Morgan and P. W. Wang, 2006. Diversifying selection drives the evolution of the type III secretion system pilus of Pseudomonas syringae. Mol. Biol. Evol. 23: 2342–2354. [DOI] [PubMed] [Google Scholar]
- Ham, J. H., D. R. Majerczak, A. S. Arroyo-Rodriguez, D. M. Mackey and D. L. Coplin, 2006. WtsE, an AvrE-family effector protein from Pantoea stewartii subsp. stewartii, causes disease-associated cell death in corn and requires a chaperone protein for stability. Mol. Plant-Microbe Interact. 19: 1092–1102. [DOI] [PubMed] [Google Scholar]
- He, S. Y., H. C. Huang and A. Collmer, 1993. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell 73: 1255–1266. [DOI] [PubMed] [Google Scholar]
- Huang, H. C., R. H. Lin, C. J. Chang, A. Collmer and W. L. Deng, 1995. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpinPss secretion that are arranged colinearly with Yersinia ysc homologs. Mol. Plant-Microbe Interact. 8: 733–746. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., and N. L. Kaplan, 1985. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111: 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguadé, 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 116: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan, H., and F. Tajima, 1997. The amounts of nucleotide variation within and between allelic classes and the reconstruction of the common ancestral sequence in a population. Genetics 147: 1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob, K., E. M. Goss, H. Araki, T. Van, M. Kreitman et al., 2002. Pseudomonas viridiflava and P. syringae–natural pathogens of Arabidopsis thaliana. Mol. Plant-Microbe Interact. 15: 1195–1203. [DOI] [PubMed] [Google Scholar]
- Jakob, K., J. Kniskern and J. Bergelson, 2007. The role of pectate lyase and the jasmonic acid defense response in Pseudomonas viridiflava virulence. Mol. Plant-Microbe Interact. 20: 146–158. [DOI] [PubMed] [Google Scholar]
- Jin, Q., and S. Y. He, 2001. Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae. Science 294: 2556–2558. [DOI] [PubMed] [Google Scholar]
- Jukes, T. H., and C. R. Cantor, 1969. Evolution of protein molecules, pp. 21–132 in Mammalian Protein Metabolism. Academic Press, New York.
- Kimura, M., 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111–120. [DOI] [PubMed] [Google Scholar]
- Kumar, S., K. Tamura, I. B. Jakobsen and M. Nei, 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17: 1244–1245. [DOI] [PubMed] [Google Scholar]
- Liu, Z. Y., J. I. Bos, M. Armstrong, S. C. Whisson, L. Da Cunha et al., 2005. Patterns of diversifying selection in the phytotoxin-like scr74 gene family of Phytophthora infestans. Mol. Biol. Evol. 22: 659–672. [DOI] [PubMed] [Google Scholar]
- Lorang, J. M., and N. T. Keen, 1995. Characterization of avrE from Pseudomonas syringae pv. tomato: a hrp-linked avirulence locus consisting of at least two transcriptional units. Mol. Plant-Microbe Interact. 8: 49–57. [DOI] [PubMed] [Google Scholar]
- McDonald, J. H., and M. Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351: 652–654. [DOI] [PubMed] [Google Scholar]
- Myers, S. R., and R. C. Griffiths, 2003. Bounds on the minimum number of recombination events in a sample history. Genetics 163: 375–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, B. C., A. Kozik, A. Griego, H. Kuang and R. W. Michelmore, 2003. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15: 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy, S. R., W. X. Huang, G. M. Weinstock and B. E. Murray, 2005. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity islands. J. Bacteriol. 187: 5709–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
- Preston, G., H. C. Huang, S. Y. He and A. Collmer, 1995. The HrpZ proteins of Pseudomonas syringae pvs. syringae, glycinea, and tomato are encoded by an operon containing Yersinia ysc homologs and elicit the hypersensitive response in tomato but not soybean. Mol. Plant-Microbe Interact. 8: 717–732. [DOI] [PubMed] [Google Scholar]
- Qiu, W. G., S. E. Schutzer, J. F. Bruno, O. Attie, X. Yun et al., 2004. Genetic exchange and plasmid transfer in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. Proc. Natl. Acad. Sci. USA 101: 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantakari, A., O. Virtaharju, S. Vahamiko, S. Taira, E. T. Palva et al., 2001. Type III secretion contributes to the pathogenesis of the soft-rot pathogen Erwinia carotovora: partial characterization of the hrp gene cluster. Mol. Plant-Microbe Interact. 14: 962–968. [DOI] [PubMed] [Google Scholar]
- Reid, S. D., C. J. Herbelin, A. C. Bumbaugh, R. K. Selander and T. S. Whittam, 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406: 64–67. [DOI] [PubMed] [Google Scholar]
- Rohmer, L., D. S. Guttman and J. L. Dangl, 2004. Diverse evolutionary mechanisms shape the type III effector virulence factor repertoire in the plant pathogen Pseudomonas syringae. Genetics 167: 1341–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas, J., J. C. Sanchez-Delbarrio, X. Messeguer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496–2497. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and M. Nei, 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Sawada, H., F. Suzuki, I. Matsuda and N. Saitou, 1999. Phylogenetic analysis of Pseudomonas syringae pathovars suggests the horizontal gene transfer of argK and the evolutionary stability of hrp gene cluster. J. Mol. Evol. 49: 627–644. [DOI] [PubMed] [Google Scholar]
- Sawyer, S. A., 1989. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 6: 526–538. [DOI] [PubMed] [Google Scholar]
- Shen, J., H. Araki, X. Sun, J.-Q. Chen and D. Tian, 2006. Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana. Genetics 172: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, E. A., G. Dwyer, R. Mauricio, M. Kreitman and J. Bergelson, 1999. Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 400: 667–671. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1993. Simple methods for testing the molecular evolutionary clock hypothesis. Genetics 135: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin and D. G. Higgins, 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, D., H. Araki, E. Stahl, J. Bergelson and M. Kreitman, 2002. Signature of balancing selection in Arabidopsis. Proc. Natl. Acad. Sci. USA 99: 11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk, K., V. C. Tam, A. R. Records, T. Petnicki-Ocwieja and J. R. Alfano, 2002. The ShcA protein is a molecular chaperone that assists in the secretion of the HopPsyA effector from the type III (Hrp) protein secretion system of Pseudomonas syringae. Mol. Microbiol. 44: 1469–1481. [DOI] [PubMed] [Google Scholar]
- Venisse, J. S., M. A. Barny, J. P. Paulin and M. N. Brisset, 2003. Involvement of three pathogenicity factors of Erwinia amylovora in the oxidative stress associated with compatible interaction in pear. FEBS Lett. 537: 198–202. [DOI] [PubMed] [Google Scholar]
- Ward, T. J., J. P. Bielawsku, H. C. Kisstler, E. Sullivan and K. O'Donnell, 2002. Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc. Natl. Acad. Sci. USA 99: 9278–9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z. M., R. J. Laby, C. H. Zumoff, D. W. Bauer, S. Y. He et al., 1992. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257: 85–88. [DOI] [PubMed] [Google Scholar]
- Xiao, Y., and S. W. Hutcheson, 1994. A single promoter sequence recognized by a newly identified alternate sigma factor directs expression of pathogenicity and host range determinants in Pseudomonas syringae. J. Bacteriol. 176: 3089–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]