Abstract
In the Saccharomyces cerevisiae strains used for genome sequencing and functional analysis, the mitochondrial DNA replicase Mip1p contains a single nucleotide polymorphism changing the strictly conserved threonine 661 to alanine. This substitution is responsible for the increased rate of mitochondrial DNA point mutations and deletions in these strains.
THE yeast Saccharomyces cerevisiae is one of the few eukaryotic organisms that can survive in the absence of mitochondrial DNA (mtDNA). When grown on glucose-containing medium, S. cerevisiae produces respiratory-deficient mutants that form small colonies and thus are referred to as “cytoplasmic petites” (Slonimski and Ephrussi 1949). They either contain deletions in their mtDNA (rho−) or are devoid of mtDNA (rho0) (Dujon 1981). petite frequency varies within a large range among laboratory strains (Marmiroli et al. 1980), a not surprising feature with regards to the numerous genes that control petite accumulation (Contamine and Picard 2000). One of these is MIP1, which encodes the mitochondrial polymerase (DNA polymerase gamma) (Foury 1989). Previous studies using the MIP1 gene isolated from a genomic library constructed with strain Σ1278b (Grenson at al. 1966), referred to as MIP1[Σ], have shown that Mip1p is an accurate replicase (Foury and Vanderstraeten 1992; Hu et al. 1995; Vanderstraeten et al. 1998). However, recent works using the MIP1 allele from S288c-related strains, referred to as MIP1[S], have pointed to higher mutation rates of the mitochondrial genome (Baruffini et al. 2006; Stuart et al. 2006). Here we show that this increase in mtDNA instability results from a single nucleotide substitution in the MIP1 gene, changing a strictly conserved threonine at position 661 to alanine.
MIP1[S] allele increases mtDNA instability:
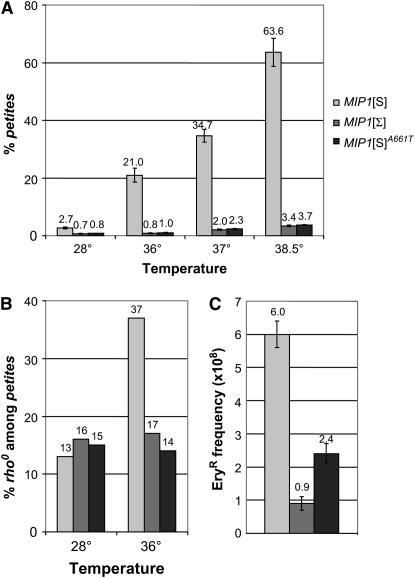
petite mutant frequency was determined in the DWM-5A-Δmip1 strain (Baruffini et al. 2006) carrying pFL39 plasmid-borne versions of either MIP1[S] or MIP1[Σ]. A fourfold increase in petite accumulation was observed at 28° in the presence of MIP1[S] compared to MIP1[Σ] (Figure 1A). Moreover, in the MIP1[S] context, and in contrast to the MIP1[Σ] context, petite accumulation was highly dependent on temperature (Figure 1A). On the basis of the capacity of independent petite clones to restore mit− mutations to wild type (Slonimski and Tzagoloff 1976), it was concluded that, in both MIP1 contexts, most petites had retained mtDNA fragments and were rho−, even though, in the MIP1[S] context, the fraction of rho0 clones at 36° was significantly higher (Figure 1B). The frequency of EryR mutants, which are caused by mtDNA mutations in the 21S rRNA gene (Sor and Fukuhara 1984), gives an estimate of the frequency of mtDNA point mutations. A sixfold increase in EryR mutants was observed in the MIP1[S] context compared to the MIP1[Σ] context (Figure 1C). These data show that in the same nuclear background the mitochondrial genome is less stable in the presence of the MIP1[S] allele, suggesting that this trait could be ascribed to differences in the amino acid sequence of the two Mip1p isoforms.
Figure 1.—
Mitochondrial DNA mutation frequency with different MIP1 alleles. DWM-5A-Δmip1, an haploid W303 derivative (Baruffini et al. 2006), was transformed with different MIP1 alleles. The MIP1 alleles were inserted into the SacI and SalI sites of the centromeric pFL39 plasmid (Bonneaud et al. 1991). The MIP1[S]A661T allele was produced by site-directed mutagenesis using the PCR overlap extension technique (Ho et al. 1989). (A) petite mutant frequency. Cells were pregrown at 28° on solid SC medium (6.7 g/liter yeast nitrogen base supplemented with a mixture of amino acids) supplemented with 2% ethanol. After 60 hr, the strains were replica plated on SC medium supplemented with 2% glucose and grown at the specified temperature. After 24 hr, strains were replica plated again on this medium. After 24 hr, cells were plated for single colonies on SC medium supplemented with 2% ethanol and 0.3% glucose. petite frequency was defined as the percentage of colonies showing the petite phenotype after 5 days at 28°. For each strain, at least 4000 clones were analyzed. Values are means of three independent experiments. (B) Percentage of rho0 mutants. The rho− clones containing mtDNA-deleted molecules and the rho0 clones devoid of mtDNA were distinguished as follows. At least 200 independent petite clones from each haploid mip1 strain were crossed with cox2, cox3, and two cob mit− mutants of opposite mating type on YPA plates (1% yeast extract, 2% bacto-peptone, and 40 mg/liter adenine) supplemented with 2% glucose, and after 2 days at 28° they were replica plated on YPA plates supplemented with 3% glycerol to identify rho+ diploids. In this work, a clone unable to complement any of the mit− mutants was arbitrarily defined as rho0. (C) EryR mutant frequency. Two independent series of 10 independent colonies grown on YPA plates supplemented with 3% glycerol were inoculated in 2.5 ml YPA medium. After 48 hr at 28°, 5–8 × 107 cells were plated on YPAEG-ery medium (YPA supplemented with 3% ethanol, 3% glycerol, 3 g/liter erythromycin, and 25 mm potassium phospate buffer at pH 6.5) and grown at 28° for 9 days. An aliquot of each culture was plated for single colonies on YPA plates supplemented with 3% glycerol to determine the exact number of rho+ cells present in the culture.
T661A substitution in the MIP1[S] allele is the cause of temperature-sensitive petite accumulation:
DNA sequence analysis revealed 26 single nucleotide polymorphisms (SNPs) between MIP1[S] and MIP1[Σ] alleles (Table 1). The MIP1 sequence was identical in W303-1B and S288c, confirming the close origin of these strains (Schacherer et al. 2007). Sixteen SNPs were silent and 10 produced nonsynonymous substitutions. Multiple amino acid alignments with several fungi and animal pol g sequences showed that these modifications affected mostly poorly conserved residues (data not shown). However, Thr661, which is found in Mip1p[Σ] and is strictly conserved in all species from yeasts to humans, is changed to alanine in Mip1p[S] due to an A-to-G transition at position 1981 of the MIP1 sequence. Moreover, Thr661 has been found in two recently sequenced S. cerevisiae strains, YJM789 (Gu et al. 2005) and RM11-1a (Lee et al. 2006), which have a MIP1[S]-like allele.
TABLE 1.
SNPs and amino acid substitutions in MIP1[Σ] and MIP1[S] alleles
| SNPa | Amino acid substitutionb | SNP | Amino acid substitution | SNP | Amino acid substitution |
|---|---|---|---|---|---|
| T23 → Cc | F8 → S | T1299 → C | Silent (P433) | A1847 → G | N616 → S |
| G103 → A | A35 → T | T1590 → C | Silent (S530) | A1981 → G | T661 → A |
| G219 → T | Silent (L73) | G1617 → A | Silent (R539) | C2166 → T | Silent (C722) |
| G627 → A | Silent (A209) | T1519 → C | M540 → T | C2932 → T | P978 → S |
| G664 → A | V222 → I | A1521 → C | N541 → H | G2957 → A | S986 → N |
| G792 → A | Silent (Q264) | T1671 → C | Silent (P557) | T3345 → C | Silent (I1115) |
| A1069 → G | K357 → E | C1680 → G | Silent (P560) | G3384 → A | Silent (E1128) |
| T1161 → A | Silent (L387) | T1692 → C | Silent (C564) | A3516 → G | Silent (P1172) |
| A1221 → G | Silent (Q407) | T1794 → G | Silent (G598) |
The first nucleotide refers to MIP1[Σ], the second to MIP1[S].
The first amino acid refers to Mip1[Σ], the second to Mip1[S].
Nonsynonymous SNPs and corresponding amino acid substitutions are in italics.
To determine whether the T661A substitution was the cause of petite accumulation in the MIP1[S] context, a MIP1[S]A661T variant was constructed. In the presence of this new allele, petite frequency was similar to that observed with the MIP1[Σ] allele and, moreover, the temperature-sensitive trait had disappeared (Figure 1A). The frequency of EryR mutants was also reduced (Figure 1C). In a heteroallelic MIP1[S]A661T/MIP1[S] strain, petite and EryR mutant frequencies were similar to those observed in a strain containing MIP1[S]A661T only, indicating that the MIP1[S] allele is recessive (data not shown).
We measured petite frequency in several laboratory strains. Σ1278b, D273-10B/A1 (Sherman 1964), and FL100 (Lacroute 1968), which possess the MIP1[Σ] allele (E. Baruffini, unpublished data), had low levels of petites (Table 2). W303-1B (Thomas and Rothstein 1989) and BY4742 (Brachmann et al. 1998), which possess the MIP1[S] allele (and thus Ala661), accumulated petites at higher levels and in a temperature-dependent manner (Table 2). To further demonstrate that increased instability of the mitochondrial genome could be ascribed to Ala661 rather than to the genetic background of these strains, the pFL38 plasmid-borne MIP1[S]A661T and MIP1[S] alleles were introduced in a mip1Δ derivative of D273-10B/A1. petite frequency was low in the presence of MIP1[S]A661T and increased in the presence of MIP1[S] (Table 3). Altogether, these data demonstrate that the T661A substitution in Mip1p is the cause of the higher accumulation of petites in S288c, BY4742, and W303-1B strains.
TABLE 2.
petite accumulation in different laboratory strains
| % petites
|
|||
|---|---|---|---|
| Strains | 28° | 36° | Amino acid at position 661 |
| D273-10B/A1 | 0.8 ± 0.1 | 0.9 ± 0.2 | Threonine |
| FL100 | 0.5 ± 0.1 | 0.8 ± 0.1 | Threonine |
| Σ1278b | 0.7 ± 0.1 | 0.8 ± 0.2 | Threonine |
| W303-1B | 2.3 ± 0.2 | 15.1 ± 2.1 | Alanine |
| BY4742 | 2.1 ± 0.2 | 11.3 ± 1.1 | Alanine |
Experimental conditions are as in Figure 1A.
TABLE 3.
petite accumulation in strain D273-CD3-Δmip1 transformed with different MIP1 alleles
| % petites
|
||
|---|---|---|
| D273-CD3-Δmip1 | 28° | 36° |
| MIP1[S] | 5.1 ± 0.4 | 22.6 ± 3.4 |
| MIP1[S]A661T | 2.2 ± 0.2 | 2.6 ± 0.3 |
D273-CD3-Δmip1 is a spontaneous ura3 mutant of D273-10B/A1 in which the MIP1 gene has been replaced by a KanR deletion cassette in the presence of the pFL38 (Bonneaud et al. 1991) plasmid-borne MIP1[S] or MIP1[S]A661T to keep mtDNA. Experimental conditions are as in Figure 1A.
Natural isolates contain a threonine residue at position 661:
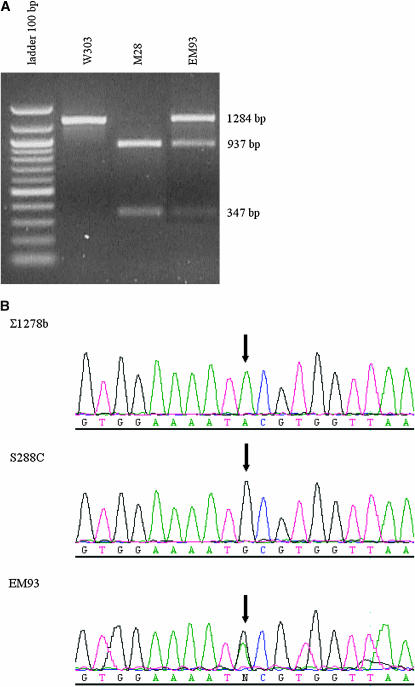
To determine whether T661A was a widespread mutation, we analyzed 20 strains isolated from grapes of different Italian regions or used as industrial starters for wine or bread production. Adenine at position 1981 of the MIP1 gene is associated with a BsaAI site while with guanine the BsaAI site is lost. To distinguish these polymorphisms, a DNA fragment encompassing nucleotides 1046–2329 was amplified and digested by BsaAI. The presence of adenine at position 1981 generates two DNA fragments of 937 and 347 bp, whereas guanine generates a single DNA fragment of 1284 bp (Figure 2A). In all strains, the MIP1 gene possessed the BsaAI site and thus Thr661.
Figure 2.—
Analysis of the polymorphism at position 1981 of the MIP1 nucleotide sequence. (A) BsaAI restriction analysis of the DNA fragment from nucleotides 1046 to 2329. All natural isolates analyzed produced the same pattern as the M28 strain, an isolate from grapes in a Tuscan vineyard (Cavalieri et al. 2000). (B) DNA sequence of the segment encompassing position 1981.
On the other hand, the partial genome sequence of haploid segregants from 36 strains of different origins has recently been published (Saccharomyces Genome Resequencing Project at the Sanger Institute, http://www.sanger.ac.uk/Teams/Team71/durbin/sgrp/index.shtml). Most Western isolates have the MIP1[S]A661T allele; the MIP1 gene of Asian isolates is a complex mosaic of the MIP1[S]A661T and MIP1[Σ] alleles, but none of the strains has the MIP1[Σ] allele, raising the question of the origin of this allele. However, all isolates have a threonine at position 661.
In contrast, analysis of the MIP1 gene of the diploid EM93 strain, which is the progenitor of S288c and derivative strains and has been estimated to share 88% of its genome with S288c (Mortimer and Johnston 1986), revealed that, in addition to the two DNA fragments generated by the BsaAI digest, an undigested fragment of 1284 bp was also present (Figure 2A), a sign of heterozygosity at the BsaA1 site. MIP1 gene sequencing and tetrad analysis (data not shown) confirmed that both A and G were present at position 1981 (Figure 2B). Therefore, EM93 is heterozygous for the MIP1 allele. These data led to the conclusion that the T661A substitution is unique and specific to EM93, the founder of several commonly used laboratory strains.
Concluding remarks:
This work provides an explanation for the high frequency of mtDNA deletions and point mutations occurring in commonly used laboratory strains: a missense mutation in the mitochondrial DNA replicase brought about by EM93, the founder strain (Mortimer and Johnston 1986). The fact that S288c and its derivatives BY4741, BY4742, and BY4743, the strains used in the genome sequencing and large-scale functional analysis projects, contain the MIP1[S] allele should be taken into consideration in genomic studies focused on alterations of the mitochondrial metabolism. Moreover, the MIP1[S] allele has previously been used to establish the impact of human pathological POLG mutations on the stability of mtDNA (Baruffini et al. 2006; Stuart et al. 2006). It must be stressed that even though this allele, which exacerbates certain defects, can be useful in detecting subtle effects of mip1 mutations, it may also cause unreliable phenotypes.
Acknowledgments
We thank I. Ferrero (University of Parma) for stimulating discussion, D. Cavalieri, M. Polsinelli (University of Florence, Italy), and M. Budroni (University of Sassari, Italy) for providing natural wine strains, and G. Liti and E. J. Louis (University of Nottingham, UK) for useful information about the S. cerevisiae strains sequenced at the Sanger Institute. We thank Roberto Silva for his skillful technical assistance. This work was funded by the Belgian National Fund for Scientific Research (to F.F.), the Interuniversity Attraction Programme, Belgian Science Policy (to F.F.), the Association Française contre les Myopathies (to F.F.), and the Fondazione Telethon-Italy no. GGP030039 and National Benefit Research Project 2006 2006069034_003 (to E.B. and T.L.).
References
- Baruffini, E., T. Lodi, C. Dallabona, A. Puglisi, M. Zeviani et al., 2006. Genetic and chemical rescue of the Saccharomyces cerevisiae phenotype induced by mitochondrial DNA polymerase mutations associated with progressive external ophthalmoplegia in humans. Hum. Mol. Genet. 15: 2846–2855. [DOI] [PubMed] [Google Scholar]
- Bonneaud, N., O. Ozier-Kalogeropoulos, G. Y. Li, M. Labouesse, L. Minvielle-Sebastia et al., 1991. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7: 609–615. [DOI] [PubMed] [Google Scholar]
- Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li et al., 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Cavalieri, D., J. P. Townsend and D. L. Hartl, 2000. Manifold anomalies in gene expression in a vineyard isolate of Saccharomyces cerevisiae revealed by DNA microarray analysis. Proc. Natl. Acad. Sci. USA 97: 12369–12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contamine, V., and M. Picard, 2000. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol. Mol. Biol. Rev. 64: 281–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon, B., 1981. Mitochondrial genetics and functions, pp. 505–635 in The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Foury, F., 1989. Cloning and sequencing of the nuclear gene MIP1 encoding the catalytic subunit of the yeast mitochondrial DNA polymerase. J. Biol. Chem. 264: 20552–20560. [PubMed] [Google Scholar]
- Foury, F., and S. Vanderstraeten, 1992. Yeast mitochondrial DNA mutators with deficient proofreading exonucleolytic activity. EMBO J. 11: 2717–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson, M., M. Mousset, J. M. Wiame and J. Bechet, 1966. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim. Biophys. Acta 127: 325–338. [DOI] [PubMed] [Google Scholar]
- Gu, Z., L. David, D. Petrov, T. Jones, R. W. Davis et al., 2005 Elevated evolutionary rates in the laboratory strains of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 102: 1092–1097. [DOI] [PMC free article] [PubMed]
- Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen and L. R. Pease, 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- Hu, J. P., S. Vanderstraeten and F. Foury, 1995. Isolation and characterization of ten mutator alleles of the mitochondrial DNA polymerase-encoding MIP1 gene from Saccharomyces cerevisiae. Gene 160: 105–110. [DOI] [PubMed] [Google Scholar]
- Lacroute, F., 1968. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J. Bacteriol. 95: 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. I., D. Pe'er, A. M. Dudley, G. M. Church and D. Koller, 2006. Identifying regulatory mechanisms using individual variation reveals key role for chromatin modification. Proc. Natl. Acad. Sci. USA 103: 14062–14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmiroli, N., F. M. Restivo, C. Donnini, L. Bianchi and P. P. Puglisi, 1980. Analysis of rho mutability in Saccharomyces cerevisiae. I. Effects of mmc and pet-ts alleles. Mol. Gen. Genet. 177: 581–588. [DOI] [PubMed] [Google Scholar]
- Mortimer, R. K., and J. R. Johnston, 1986. Genealogy of principal strains of the yeast genetic stock center. Genetics 113: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacherer, J., D. M. Ruderfer, D. Gresham, K. Dolinski, D. Botstein et al., 2007. Genome-wide analysis of nucleotide-level variation in commonly used Saccharomyces cerevisiae strains. PLoS ONE 3: e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., 1964. Mutants of yeast deficient in cytochrome c. Genetics 49: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonimski, P. P., and B. Ephrussi, 1949. Action de l'acriflavine sur les levures. V. Le système des cytochromes des mutants “petite colonie.” Ann. Inst. Pasteur (Paris) 77: 47–63. [Google Scholar]
- Slonimski, P. P., and A. Tzagoloff, 1976. Localization in yeast mitochondrial DNA of mutations expressed in a deficiency of cytochrome oxidase and/or coenzyme QH2-cytochrome c reductase. Eur. J. Biochem. 61: 27–41. [DOI] [PubMed] [Google Scholar]
- Sor, F., and H. Fukuhara, 1984. Erythromycin and spiramycin resistance mutations of yeast mitochondria: nature of the rib2 locus in the large ribosomal RNA gene. Nucleic Acids Res. 12: 8313–8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, G. R., J. H. Santos, M. K. Strand, B. Van Houten and W. C. Copeland, 2006. Mitochondrial and nuclear DNA defects in Saccharomyces cerevisiae with mutations in DNA polymerase γ associated with progressive external ophthalmoplegia. Hum. Mol. Genet. 15: 363–374. [DOI] [PubMed] [Google Scholar]
- Thomas, B. J., and R. Rothstein, 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56: 619–630. [DOI] [PubMed] [Google Scholar]
- Vanderstraeten, S., S. Van Den Brule, J. P. Hu and F. Foury, 1998. The role of 3′-5′ exonucleolytic proofreading and mismatch repair in yeast mitochondrial DNA error avoidance. J. Biol. Chem. 273: 23690–23697. [DOI] [PubMed] [Google Scholar]