Abstract
Gene flow occurs predominantly via pollen in angiosperms, leading to stronger population subdivision for maternally inherited markers, relative to paternally or biparentally inherited genes. In contrast to this trend, population subdivision within Silene latifolia and S. dioica, as well as subdivision between the two species, is substantially lower in maternally inherited chloroplast genes compared to paternally inherited Y-linked genes. A significant frequency spectrum bias toward rare polymorphisms and a significant loss of polymorphism in chloroplast genes compared to Y-linked and autosomal genes suggest that intra- and inter-specific subdivision in the chloroplast DNA may have been eroded by a selective sweep that has crossed the S. latifolia and S. dioica species boundary.
CHLOROPLAST DNA (cpDNA) is widely used in studies of plant phylogeography. Often such studies do not use any additional data from nuclear genes (e.g., Taylor and Keller 2007) and thus implicitly or explicitly assume that the gene tree of chloroplast sequences reflects the history and population dynamics of the species. However, non-neutrality in chloroplast genes may significantly affect the tree structure and bias the inferences of biogeographic studies based on cpDNA-linked polymorphisms. The chloroplast genome includes many genes that may have a direct effect on plant growth. For example, the chloroplast gene rbcL that is often used in plant systematics encodes a large subunit of the RUBISCO enzyme that is a critical component of photosynthesis and one of the limiting steps in plant growth (Spreitzer and Salvucci 2002). It was recently demonstrated that positive selection in rbcL is fairly widespread in terrestrial plants (Kapralov and Filatov 2007). The spread of adaptive mutations in rbcL or any other chloroplast gene will affect the distribution of polymorphisms all over the chloroplast genome and may result in a strong bias for phylogeographic studies based only on cpDNA. Moreover, adaptive cytotypes may spread across several hybridizing species (e.g., Tsitrone et al. 2003; Seehausen 2004). This may create incongruencies between chloroplast-based phylogenies and the ‘true’ species phylogeny. For example, adaptive selection in the RUBISCO enzyme in the Hawaiian endemic genus Schiedea may have driven the spread and fixation of adaptive cytotypes in several Schiedea species inhabiting the same island of the archipelago. This in turn created a strong incongruence between the chloroplast gene tree and the phylogeny of the genus (Kapralov and Filatov 2006). Thus, using only chloroplast DNA for phylogenetic and phylogeographic purposes may be misleading.
Here we report a study of a selective sweep in chloroplast DNA across the range of two dioecious Silene species, Silene latifolia and S. dioica (Caryophyllaceae). These two species are closely related and belong to a small cluster of dioecious Silene species (section Elisanthe), which includes S. latifolia, S. dioica, S. heuffelii, S. diclinis, and S. marizii. All these species are closely related and can form viable and fertile hybrids (except S. latifolia × S. diclinis crosses that rarely produce seeds; Prentice 1978) and are known to have cytologically distinguishable sex chromosomes. The range of S. latifolia in Eurasia stretches from Britain and Scandinavia down to northern Morocco reaching as far as the Black Sea to the south and Lake Baikal in the east (Baker 1947). S. dioica occurs throughout northern Europe, where its natural range broadly overlaps that of S. latifolia. S. heuffelii occurs in the northern Balkan region and the Carpathian Mountains at altitudes >700–800 m while S. marizii has an extremely disjunct distribution, occurring in central Spain and northern Portugal. S. diclinis is an endemic species, occupying an extremely restricted geographical range surrounding the town of Xativa in the Spanish province of Valencia (Prentice 1976).
The earlier studies of allozyme (Mastenbroek et al. 1984) and flavenoid variation (Mastenbroek and Van Brederode 1986) and pollen/seed morphology indicate that European S. latifolia populations are divided into nine geographical races with a major east–west subdivision (Mastenbroek and Van Brederode 1986). At the local level, S. latifolia populations (as well as populations of many other Silene species) are also fairly structured, with plants growing in small demes that follow metapopulation dynamics. Since gene flow in S. latifolia occurs predominantly via pollen (Mccauley 1997), chloroplast genes (transmitted via seed) are expected to be particularly affected by population structure (e.g., Ennos 1994), whereas paternally inherited markers are expected to be the least structured (Laporte and Charlesworth 2002). Surprisingly, paternally inherited Y-linked genes show very high levels of population structure [Wright's FST statistic (Nei 1987) is ∼ 0.9] in S. latifolia populations and the causes of such extreme structuring are not clear (Ironside and Filatov 2005). Here we report the analysis of DNA sequence polymorphism and population subdivision in the chloroplast DNA of S. latifolia and S. dioica and in a newly isolated autosomal gene and compare their population structure to that in previously studied Y- and X-linked genes.
Chloroplast population structure in S. latifolia has previously been studied in American populations by Mccauley (1994) but at a fairly local scale (<25 km between populations). In this instance, chloroplast DNA length variants were highly structured (FST ∼ 0.67) and exhibited significantly greater levels of population structure than that observed for biparentally inherited allozymes (FST ∼ 0.13). Chloroplast DNA diversity in S. latifolia has been previously characterized by Ingvarsson and Taylor (2002), but population structure was not analyzed, probably because their sample size (25 individuals) and sampling scheme were unsuitable for this purpose. A recent study by the same group (Taylor and Keller 2007) looked at S. latifolia population structure and demography, but as we show later, the conclusions of this article may have been biased by a selective sweep in S. latifolia cpDNA.
Our sample includes 75 S. latifolia and 29 S. dioica collected from across Eurasia. In addition we also included individuals from other dioecious Elisanthe species: S. heuffelii, S. diclinis, and S. marizii. The range of these endemic species is fairly restricted and intraspecific polymorphism is relatively low (Prentice 1984); hence, we included only one-two individuals of each of these three species. Given the evidence for hybridization between S. latifolia and S. dioica (Baker 1948) and the possibility of interspecific gene flow among other species in the section Elisanthe (Prentice 1978), all five dioecious species in the Elisanthe section may be treated as a single metapopulation. Thus, in the analyses presented below we treat all five dioecious Silene species both separately and jointly (the latter referred to as Elisanthe). When the species are considered separately, we analyze only S. latifolia and S. dioica because samples from the other three dioecious species are insufficient for a population level analysis. The exact locations of the samples used in this study are listed in the supplemental Table S1 at http://www.genetics.org/supplemental/.
DNA POLYMORPHISM IN SILENE cpDNA AND A NEW AUTOSOMAL GENE SLOP
To characterize polymorphism and the extent of population subdivision in the chloroplast DNA of Elisanthe species, we PCR amplified and sequenced three regions of the chloroplast genome: (i) a 1.8-kb region between the trnT and trnF genes, including the trnL gene and its intron; (ii) a 1-kb fragment of the matK gene; and (iii) a 1.3-kb fragment of the rbcL gene (Table 1). The primers used for amplification and sequencing are listed in the supplemental Table S2 at http://www.genetics.org/supplemental/. In addition to Elisanthe samples, we sequenced the same cpDNA regions from two outgroup species, S. conica and S. vulgaris. Although S. vulgaris is regarded as the more distant relative of the two, this species demonstrates lower sequence divergence from the Elisanthe group than S. conica for chloroplast genes (e.g., matK, 3.7 and 7.7%, for S. vulgaris and S. conica, respectively), an observation that may result from a higher substitution rate in S. conica (data not shown). For this reason, we chose to use S. vulgaris as an outgroup (Figure 1).
TABLE 1.
Summary of DNA diversity in S. latifolia and S. dioica chloroplast and autosomal genes
| All sites
|
Nonsilenta
|
Silentb
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Taxon | Nc | Length | Sd | π(%)e | θ (%)f | Dg | HKAh | Length | Sd | π(%)e | θ (%)f | Length | Sd | π(%)e | θ (%)f |
| Chloroplast | ||||||||||||||||
| TrnTLF | Elisanthe | 92 | 1644 | 31 | 0.165 | 0.37 | −1.71 | NS | 55 | 0 | 0 | 0 | 1589 | 31 | 0.171 | 0.383 |
| S. latifolia | 65 | 1645 | 19 | 0.136 | 0.243 | −1.34 | NS | 55 | 0 | 0 | 0 | 1590 | 19 | 0.141 | 0.252 | |
| S. dioica | 23 | 1655 | 17 | 0.186 | 0.278 | −1.07 | NS | 55 | 0 | 0 | 0 | 1600 | 17 | 0.118 | 0.288 | |
| MatK | Elisanthe | 101 | 1014 | 27 | 0.18 | 0.51 | −1.95* | P = 0.0029 | 788.7 | 15 | 0.115 | 0.367 | 225.3 | 12 | 0.411 | 1.03 |
| S. latifolia | 69 | 1014 | 18 | 0.14 | 0.37 | −1.86* | P = 0.0008 | 788.72 | 11 | 0.077 | 0.29 | 225.28 | 7 | 0.362 | 0.647 | |
| S. dioica | 27 | 1014 | 14 | 0.22 | 0.36 | −1.29 | P = 0.0007 | 788.65 | 6 | 0.186 | 0.197 | 225.35 | 8 | 0.354 | 0.921 | |
| RbcL | Elisanthe | 69 | 1314 | 6 | 0.02 | 0.1 | −1.80* | NS | 992.02 | 1 | 0.006 | 0.021 | 321.98 | 5 | 0.078 | 0.323 |
| S. latifolia | 50 | 1314 | 2 | 0.02 | 0.03 | −1.01 | NS | 992.01 | 1 | 0.008 | 0.023 | 321.99 | 1 | 0.036 | 0.069 | |
| S. dioica | 15 | 1314 | 3 | 0.04 | 0.07 | −1.32 | NS | 992.04 | 0 | 0 | 0 | 321.96 | 3 | 0.16 | 0.287 | |
| TrnTLF | Elisanthe | 87 | 2658 | 53 | 0.171 | 0.396 | −1.87* | P = 0.033 | 843.7 | 13 | 0.114 | 0.327 | 1799.6 | 40 | 0.202 | 0.441 |
| + MatK | S. latifolia | 62 | 2659 | 35 | 0.141 | 0.28 | −1.63* | P = 0.0135 | 843.73 | 9 | 0.077 | 0.243 | 1800.6 | 26 | 0.174 | 0.307 |
| S. dioica | 22 | 2669 | 29 | 0.202 | 0.298 | −1.24 | P = 0.0061 | 843.64 | 6 | 0.199 | 0.209 | 1809.7 | 23 | 0.211 | 0.349 | |
| Autosomal | ||||||||||||||||
| Slop | Elisanthe | 70 | 2198 | 146 | 0.696 | 1.43 | −1.76 | 166 | 3 | 0.05 | 0.27 | 2028 | 148 | 0.75 | 1.51 | |
| S. latifolia | 42 | 2221 | 93 | 0.607 | 1.01 | −1.44 | 166.02 | 2 | 0.06 | 0.2 | 2055 | 94 | 0.65 | 1.06 | ||
| S. dioica | 28 | 2312 | 75 | 0.554 | 0.845 | −1.39 | 165.97 | 1 | 0.03 | 0.11 | 2146 | 75 | 0.59 | 0.91 | ||
DNA diversity statistics and neutrality tests were conducted in the programs: ProSeq (Filatov 2002) and DnaSP (Rozas et al. 2003).
For the trnL gene encoding tRNA for leucine, all positions in exons are counted as non-silent.
Silent sites: synonymous and noncoding.
Sample size.
Number of polymorphic sites.
Average heterozygosity per 100 sites (Nei 1987, equation 10.5).
Watterson's estimator of scaled mutation rate (2Neμ) per 100 sites (Watterson 1975).
Tajima's D (Tajima 1989), significance or rejection of neutrality is indicated as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
Comparison of the amount of polymorphism in the chloroplast genes and the autosomal Slop gene by the HKA test of neutrality (Hudson et al. 1987).
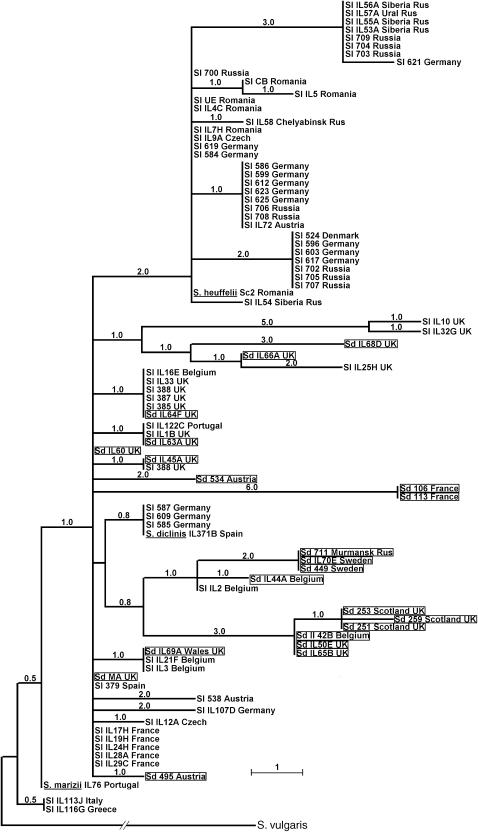
Figure 1.—
Neighbor-joining tree of dioecious Silene (section Elisanthe) chloroplast sequences (concatenated matK + trnT-trnL-trnF) rooted by an outgroup S. vulgaris. S. dioica individuals are framed and S. diclinis, S. heuffelii, and S. marizii are underlined. The lengths of individual branches are shown in terms of the number of substitutions along the branch.
The two protein-coding genes studied, matK and rbcL, differ dramatically in the amount of DNA polymorphism segregating in these genes. matK was relatively polymorphic, with 12 synonymous and 15 nonsynonymous polymorphisms found in the entire Elisanthe data set. DNA diversity in the rbcL gene was substantially lower, however, with only six polymorphic sites (five synonymous mutations and one replacement mutation) being detected in the entire sample from five dioecious Silene species (Table 1). Low DNA diversity in the rbcL gene provided little information regarding population structure; hence, this gene was not sequenced from all individuals used in the study and it was not included in the concatenated data set. The trnT-trnL-trnF region studied includes two exons of the trnL gene, an intron between the trnL exons, as well as intergenic spacers between the trnT-trnL and trnL-trnF genes. No nucleotide polymorphisms were detected in the exons of the trnL gene. The trnL intron is less polymorphic (π = 0.083% for the entire Elisanthe data set) compared to intergenic regions (π = 0.22% for the same data set), suggesting that the intron is under purifying selective constraint to maintain RNA structure essential for splicing (Cech 1990).
Intraspecific levels of polymorphism in the noncoding regions of the chloroplast DNA were somewhat lower (π ∼ 0.2%; Table 1) than DNA diversity observed in noncoding regions of the Y chromosome (π = 0.67%; see Ironside and Filatov 2005). However, the difference may reflect lower mutation rates in the chloroplast compared to nuclear genes. We used sequences of the trnT-trnL and trnL-trnF intergenic spacers and of an intron of the DD44 gene from S. vulgaris to correct for differences in mutation rates between the chloroplast and the Y chromosome. The mutations in noncoding DNA should be predominantly neutral, so the rate of divergence among the species is proportional to the mutation rate. When the diversity differences between the Y-linked and the chloroplast genes were corrected for their divergence from homologs in S. vulgaris, the difference was not significant (HKA test, P > 0.05; Hudson et al. 1987), suggesting that the observed polymorphism difference between the nuclear and chloroplast genes may be explained by differences in mutation rates.
For a comparison with an autosomal locus we sequenced 70 Elisanthe individuals for a fragment of a new non-sex-linked gene, Slop, which was isolated from a S. latifolia male flower bud cDNA library as a by-product of a search for new sex-linked genes (reviewed in Filatov 2005). This gene showed a segregation pattern incompatible with sex-linkage and was considered therefore to be autosomal (data not shown). The gene encodes a protein with strong homology to Arabidopsis oligopeptidase. Accordingly, we named it Slop (S. latifolia oligopeptidase).
Using the primers listed in supplemental Table S2 at http://www.genetics.org/supplemental/, we PCR-amplified a 2.3-kb region of the Slop gene from 42 S. latifolia, 28 S. dioica, 1 S. diclinis, 1 S. marizii, and 1 S. vulgaris. PCR products were sequenced directly, resulting in diploid unphased sequences. To establish the phase we cloned PCR products and sequenced the clones in addition to PCR products. Although it is possible from such data to reconstruct two phased copies of a gene from each individual, we have not done this and used only one phased allele per individual, as this was sufficient for our purposes. Average heterozygosity in the Slop intron was 0.65 and 0.59% in S. latifolia and S. dioica, respectively (Table 1), significantly higher than in the chloroplast DNA in the two species separately and in the pooled Elisanthe data set (HKA test, P < 0.003). This test takes into account ploidy and mutation rate differences between the genes compared (Hudson et al. 1987). The result suggests that either the Slop gene has unusually high DNA diversity or that diversity in the chloroplast DNA is significantly reduced. DNA diversity in Slop is actually lower than in the X-linked genes reported previously (Filatov et al. 2000, 2001; Ironside and Filatov 2005); thus it seems unlikely that the significant HKA is due to elevated levels of polymorphism in the autosomal gene.
The HKA test assumes that sequences follow a neutral coalescent process in which polymorphism is proportional to divergence, which may not be true for subdivided Silene populations. However, Wakeley (1999) has shown that the coalescent approach may still be useful in subdivided populations, if only one allele is sampled from a subpopulation or deme. In this study we sampled one allele from as many demes as possible; thus, the subdivision in Silene populations should not affect the results of the HKA test.
A SELECTIVE SWEEP IN S. LATIFOLIA AND S. DIOICA CHLOROPLAST DNA
The reduced DNA diversity in the chloroplast DNA, compared to the autosomal and X-linked loci of both S. latifolia and S. dioica, as well as the two species pooled together suggest a scenario of a selective sweep in the chloroplast (or a linked mitochondrial) genome across the range of the two species. If this were the case, one would expect such a sweep to bias the frequency spectrum of mutations toward rare polymorphisms. Indeed, Tajima's D (Tajima 1989) is consistently negative across each 1 of the 3 chloroplast genes, and neutrality can be rejected at the 5% level in Elisanthe and S. latifolia matK and joint matK and trnTLF data sets, as well as for Elisanthe rbcL, demonstrating an excess of low-frequency polymorphisms and indicating either a selective sweep in the chloroplast or population expansion. These results contrast with those observed for Y-linked genes, where high-frequency polymorphisms are in significant excess, with X-linked genes (Filatov et al. 2000, 2001; Ironside and Filatov 2005; Laporte et al. 2005) and with the autosomal Slop gene, where no significant frequency spectrum bias was detected in either of the species. These observations suggest that a demographic scenario is unlikely, as demographic processes are expected to affect all genes across all genomes. The significantly negative Tajima's D for the cpDNA of S. latifolia and S. dioica can not be the result of subdivided population structure in these species because we sampled one allele per deme, and a genealogy of such a sample is still a coalescent despite the population subdivision (Wakeley 1999). Furthermore, population subdivision is expected to bias Tajima's D toward positive values; hence, the observed significantly negative values may be underestimates and are conservative.
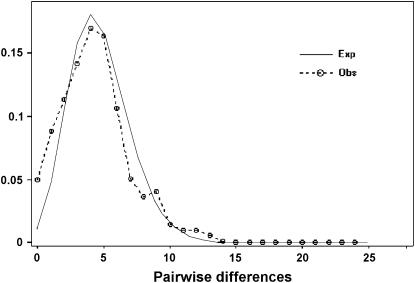
The distribution of the observed number of differences between pairs of haplotypes (mismatch distribution) can be used to estimate the time since population expansion or a selective sweep (Rogers and Harpending 1992). At demographic equilibrium this distribution is usually multimodal, but it is unimodal in populations having passed through a recent demographic expansion (Slatkin and Hudson 1991; Rogers and Harpending 1992). The unimodal distribution of the mismatch distribution in Silene chloroplast genes is consistent with population growth or a selective sweep (Figure 2). It is possible to fit the model of exponential population growth to the data to estimate the time of population expansion or the selective sweep. The model has three parameters: scaled mutation rates before (θb) and after (θa) growth, and τ is the time since population expansion measured in units of mutational time (τ = 2ut, where t is the time in generations, and u is the mutation rate per sequence and per generation; Rogers and Harpending 1992). Assuming that θa is infinite, Rogers (1995) derived a moment estimator of the time since population expansion (τ). This value, calculated using the program DnaSP (Rozas et al. 2003), is similar for S. latifolia and S. dioica data sets analyzed separately and for the pooled Elisanthe data set (2.59, 4.0, and 2.99, respectively).
Figure 2.—
Distribution of pairwise differences between sequences in the Silene section Elisanthe data set (concatenated matK + trnT-trnL-trnF data set). The expected distribution was generated using DnaSP (Rozas et al. 2003) for the model of exponential population growth, which started τ = (2μt) = 3.98 generations ago (Rogers and Harpending 1992).
Schneider and Excoffier (1999) found that the moment estimator often underestimates the age of the expansion/sweep. Using their generalized nonlinear least-square approach implemented in ARLEQUIN 3.01 (Excoffier et al. 2005), the estimates of τ are slightly higher than those obtained by the moment estimator. For S. latifolia, S. dioica, and the pooled Elisanthe data set the time since expansion/sweep is τ = 4.157 (1% CI: 2.708–5.340), 6.208 (1% CI: 4.158–9.103), and 3.983 (1% CI: 2.725–8.200), respectively. As before, the estimate of time since growth/sweep (τ = 2ut) is measured in mutational units. Assuming a mutation rate of 1.1–2.9 × 10−9 nucleotide substitutions per site per year (Wolfe et al. 1987; Clegg et al. 1994), the selective sweep in the chloroplast DNA may have happened between 0.16 [=τ /2uL= 2.5/(2 × 2.9 × 10−9 × 2660 bp)] and 1.06 [=τ /2uL= 6.2/(2 × 1.1 × 10−9 × 2660 bp)] MYA.
The frequency spectrum bias toward rare polymorphisms can be caused either by a population expansion or a selective sweep in the chloroplast genome. However, for the autosomal Slop gene Tajima's D is not significantly negative in either of the species and no bias toward rare polymorphisms was detected in S. latifolia Y- or X-linked genes (Filatov et al. 2000, 2001; Ironside and Filatov 2005; Laporte et al. 2005), suggesting that a selective sweep scenario is a more likely explanation for the patterns observed in the chloroplast. The rate of loss of bias in the frequency spectrum depends on the effective population size, with larger populations taking longer to return to equilibrium (e.g., Przeworski 2002). As nuclear genes have larger effective population sizes, on average they should have deeper genealogies (or older most recent common ancestors) and are expected to retain the footprint of older population expansions compared to chloroplast DNA. The absence of a population expansion signal, thus far, for nuclear genes gives strong support against the population expansion model in favor of a chloroplast selective sweep. Human-mediated range expansion, such as is thought to have occurred in S. latifolia with the advent of agriculture (<10,000 years ago), is probably too recent to be detectable from slowly mutating DNA sequences.
A recent study by Taylor and Keller (2007) used chloroplast DNA sequences to infer the phylogeography of S. latifolia and S. vulgaris. As only chloroplast sequences were used, they could not distinguish between the effects of selection and demography, which led to the conclusion that S. latifolia may have undergone population expansion around 0.6 MYA. Our data demonstrate that, in fact, the signal of population expansion around that time was created by a chloroplast-specific selective sweep and not population expansion. This illustrates the importance of a multigenic approach in experimental population genetics and phylogeography.
LOW POPULATION STRUCTURE IN S. LATIFOLIA AND S. DIOICA CHLOROPLAST DNA
Surveys of chloroplast population structure are expected to exceed those for the Y chromosome and for autosomal genes, reflecting the reduced levels of gene flow per generation via seed compared to pollen (e.g., Ennos 1994). For the analyses of population subdivision we used the concatenated data set of matK and trnT-trnL-trnF genes, which included 62 S. latifolia and 22 S. dioica individuals. Population subdivision in the cpDNA was compared to the autosomal gene Slop and to the Y-linked gene DD44Y. S. latifolia and S. dioica samples were divided into populations according to country of origin of the samples.
To characterize population structure at different hierarchical levels (species and geographical races), we conducted an analysis of molecular variance (AMOVA) (Excoffier et al. 1992) as implemented in ARLEQUIN 3.01 (Excoffier et al. 2005). Despite a significant component of variation both between species and among populations, most of the variation (52.1%) in the chloroplast DNA is partitioned within populations (Table 2). A comparative analysis for the Y chromosome, however, shows a pattern diametrically opposed to that observed in the chloroplast. Only 3.8% of variation is partitioned within populations, while most of the Y chromosome diversity is partitioned among populations and species (Table 2). This result also holds when S. dioica samples are excluded from the analysis (Table 2). In parallel with the chloroplast DNA, the autosomal Slop gene demonstrated little subdivision within S. latifolia, with 99.8% of variation partitioned within populations. Thus, strong population structure in the Y chromosome reported by Ironside and Filatov (2005) contrasts with both the chloroplast and autosomal data and may be caused by some form of selection on the Y chromosome. For example, local adaptation of Y-linked genes may drastically reduce the migration rate of Y chromosomes between populations, resulting in strong population subdivision. The causes of strong population subdivision on the Silene Y chromosome require further investigation.
TABLE 2.
AMOVA analysis of population structure in the chloroplast, autosomal and Y-linked genes in Silene latifolia and S. dioica
| Chloroplast DNAa
|
Autosomal Slopb
|
Y-linked DD44Yc
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | Variation component | % variation | Φd | d.f. | Variation component | % variation | Φd | d.f. | Variation component | % variation | Φd | |
| S. latifolia and S. dioica | ||||||||||||
| Between species | 1 | 0.4 | 15.2 | ΦCT = 0.152* | 1 | 2.951 | 32.07 | ΦCT = 0.321*** | 1 | 14.99 | 76.03 | ΦCT = 0.76* |
| Among populations/species | 12 | 0.866 | 32.8 | ΦSC = 0.386*** | 11 | 0.085 | 0.92 | ΦSC = 0.014 | 6 | 3.986 | 20.21 | ΦSC = 0.84*** |
| Within populations | 70 | 1.375 | 52.1 | ΦST = 0.479*** | 59 | 6.167 | 67.01 | ΦST = 0.33*** | 27 | 0.743 | 3.77 | ΦST = 0.96*** |
| S. latifolia | ||||||||||||
| Between populations | 7 | 0.578 | 29.4 | 6 | 0.013 | 0.2 | 5 | 4.39 | 85 | |||
| Within races | 54 | 1.388 | 70.6 | ΦST = 0.294*** | 36 | 6.447 | 99.8 | ΦST = 0.011 | 24 | 0.72 | 14 | ΦST = 0.86*** |
Data from this study, concatenated matK and trnT-trnL-trnF data set, see Table 1.
Data from this study, the autosomal Slop gene, see Table 1.
Data from Ironside and Filatov (2005).
Φ is a fixation index similar to Wright's F statistics. It reflects the correlation of random pairs of haplotypes drawn from a group (species or race) relative to the correlation of pairs of random haplotypes drawn from the whole data set (Excoffier et al. 1992). Significance is indicated with stars: ***P < 0.001; **P < 0.01; *P < 0.05.
POPULATION SUBDIVISION AT THE LOCAL AND CONTINENTAL SCALES
The relatively low population subdivision in the chloroplast DNA at the continental scale that is reported in this study contrasts with the relatively strong subdivision at the local scale for the two species (Mccauley 1994; Ingvarsson and Giles 1999). The difference between the studies may be due to differences in habitat or differences between European and North American populations. Alternatively, the difference may result from population differentiation occurring faster in populations with small rather than large effective population sizes (FST being inversely related to effective population size; Wright 1931; Slatkin and Voelm 1991; Vigouroux and Couvet 2000). With little gene flow, small local populations become differentiated fairly rapidly due to genetic drift. FST among races and species, which have much larger effective population sizes, grows much slower than at the local scale. Thus, the strong differentiation at the local scale detected by Mccauley (1994) and Ingvarsson and Giles (1999) may reflect low historical levels of gene flow among demes, while the subdivision among races and species analyzed in this study may reflect gene flow and drift dynamics in larger populations over a longer time scale.
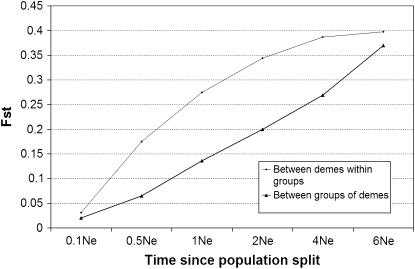
The theoretical analysis of population structure in a hierarchical island model has been conducted by Slatkin and Voelm (1991) and Vigouroux and Couvet (2000). However, these studies focused on equilibrium populations, which may not be applicable to S. latifolia and S. dioica cpDNA, where population structure may have been eliminated by a selective sweep. To illustrate the dynamics of FST for a nonequilibrium subdivided population that has lost population structure T generations ago (measured in terms of effective population size, Ne), we conducted coalescent simulations in a hierarchical island model with two groups of two demes (Figure 3). As the effective population size of a combined group of demes is larger than for separate demes, the population differentiation (FST) for separate demes grows more rapidly than at the group level. Note that this disparity occurs even in the face of higher gene flow at the level of the deme (fivefold difference) compared to the group level (Figure 3). Although we have not exhaustively explored the parameter space, it is clear that in a subdivided population that has lost population differentiation (in a certain gene T generations ago; say, due to a selective sweep), FST among small demes will build up more quickly than among groups of demes with larger combined effective population size.
Figure 3.—
Population differentiation in a hierarchical island model for two groups of two demes. Polymorphism data was generated by coalescent simulations using the ms program (Hudson 2002). To calculate FST for the simulated data sets we imported the output of ms into ProSeq v3 (Filatov 2002). The simulations assumed that all demes were of equal size with θ = 2 and 10 sequences were sampled from each deme. Gene flow was 5 times higher for demes within a group (4Nm = 0.5) than between the 2 groups (4Nm = 0.1). Going backward in time, at time T (measured in Ne generations) all 4 demes merge into a single deme. Dots represent differentiation between demes within a group of demes and triangles represent differentiation between groups of demes.
INTERSPECIFIC SELECTIVE SWEEP
The presence of a footprint of selection in both species raises the question of the scale and scenario proposed for the selective sweep. Did the sweeps occur independently in each species? Alternatively, did the same sweep cross the species boundary?
At the species level, there is significant (ΦCT = 0.321, P < 0.001) subdivision between S. latifolia and S. dioica for the autosomal Slop gene, with 32% of variation partitioned between the 2 species (Table 2). This contrasts with relatively little subdivision between the two species in chloroplast DNA (15.2% of variation is partitioned between the species), which is only marginally significant (ΦCT = 0.152, P < 0.05). Two independent sweeps in S. latifolia and S. dioica would drastically inflate FST (and ΦCT) between the two species; thus, this scenario seems unlikely. A sweep that has crossed the S. latifolia/S. dioica species boundary should have reduced differentiation between the two species, which is indeed observed in the cpDNA. As the two species hybridize naturally in contact zones, a globally adaptive mutation in the chloroplast or mitochondrial genome, which spreads across the species boundary, could have wiped out population and species-specific differentiation in cytoplasmic DNA. Following such a sweep, population differentiation within species will build up more quickly than differentiation between species because the effective population sizes of the two species are probably much larger than those constituting races and local populations. In line with this prediction, population differentiation between S. latifolia and S. dioica cpDNA is marginally significant at the species level, while subdivision among S. latifolia races and between East/West European groups shows highly significant population structure (Table 2).
Another factor that may have contributed to the relatively low differentiation between S. latifolia and S. dioica is frequent haplotype sharing between the two species, which is evident in Figure 1. Haplotype sharing can either result from gene flow between species or from shared ancestral polymorphisms segregating in both species. If gene flow is the cause of haplotype sharing, then one would expect to see shared haplotypes to be mainly present in geographic regions where the ranges of S. latifolia and S. dioica overlap. If shared ancestry is the cause, then shared haplotypes should be present across entire species range. In fact, haplotype sharing is most common in the UK and Belgium, the places where the two species coexist and often form hybrids, but no haplotypes are shared in localities where S. dioica is quite rare or absent (Eastern Europe/Russia; Figure 1). This geographical pattern suggests that shared haplotypes arose through interspecific hybridization in regions of sympatry. Interspecific gene flow may be typical for other Elisanthe species: S. heuffelii, a native of the Carpathian Mountains in Romania, clusters with East European and Russian samples, while the two species native to the Iberian Peninsula (S. marizii and S. diclinis) cluster with West European samples (Figure 1).
CONCLUSIONS
We have reported a selective sweep in the chloroplast DNA of two closely related dioecious species, S. latifolia and S. dioica, which may have occurred between 0.16 and 1.06 MYA. The signature of this sweep may be confused with the signal of population expansion if only chloroplast genome sequences are used in the analysis. The adaptive cytotype may have crossed the species boundary and spread across the range of the two species, wiping out divergence between the two species and population structure within species in the chloroplast DNA. Such sweeps of adaptive mutations between hybridizing species may be common, especially in plants, playing an important role in adaptation and speciation.
Acknowledgments
We thank Jagdeesh Jagpal and Abdul Mumtaz for contributing several S. latifolia trnL sequences, the handling editor, Outi Savolainen, and the anonymous reviewers for helpful suggestions. This work was supported by an NERC grant to D.F.
References
- Baker, H. G., 1947. Biological flora of the British Isles: Melandrium (Roehling em.). Fries. J. Ecol. 35: 271–292. [Google Scholar]
- Baker, H. G., 1948. Stages in invasion and replacement demonstrated by species of Melandrium. J. Ecol. 36: 96–119. [Google Scholar]
- Cech, T. R., 1990. Self-splicing of group-I introns. Ann. Rev. Biochem. 59: 543–568. [DOI] [PubMed] [Google Scholar]
- Clegg, M. T., B. S. Gaut, G. H. Learn, Jr. and B. R. Morton, 1994. Rates and patterns of chloroplast DNA evolution. Proc. Natl. Acad. Sci. USA 91: 6795–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennos, R. A., 1994. Estimating the relative rates of pollen and seed migration among plant populations. Heredity 72: 250–259. [Google Scholar]
- Excoffier, L., P. E. Smouse and J. M. Quattro, 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier, L., G. Laval and S. Schneider, 2005. Arlequin ver. 3.1: an integrated software package for population genetics data analysis. Evol. Bioinfo. Online 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- Filatov, D. A., 2002. ProSeq: a software for preparation and evolutionary analysis of DNA sequence data sets. Mol. Ecol. Notes 2: 621–624. [Google Scholar]
- Filatov, D. A., 2005. Isolation of genes from plant Y chromosomes. Methods Enzymol. 395: 418–442. [DOI] [PubMed] [Google Scholar]
- Filatov, D. A., F. Moneger, I. Negrutiu and D. Charlesworth, 2000. Low variability in a Y-linked plant gene and its implications for Y-chromosome evolution. Nature 404: 388–390. [DOI] [PubMed] [Google Scholar]
- Filatov, D. A., V. Laporte, C. Vitte and D. Charlesworth, 2001. DNA diversity in sex-linked and autosomal genes of the plant species Silene latifolia and Silene dioica. Mol. Biol. Evol. 18: 1442–1454. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., 2002. Generating samples under a Wright-Fisher neutral model of genetic variation. Bioinformatics 18: 337–338. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguade, 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 116: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson, P. K., and B. E. Giles, 1999. Kin-structured colonisation and small-scale genetic differentiation in Silene dioica. Evolution 53: 605–611. [DOI] [PubMed] [Google Scholar]
- Ingvarsson, P. K., and D. R. Taylor, 2002. Genealogical evidence for epidemics of selfish genes. Proc. Natl. Acad. Sci. USA 99: 11265–11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside, J. E., and D. A. Filatov, 2005. Extreme population structure and high interspecific divergence of the Silene Y chromosome. Genetics 171: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapralov, M. V., and D. A. Filatov, 2006. Molecular adaptation during adaptive radiation in the Hawaiian endemic genus Schiedea. PLoS One 1: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapralov, M. V., and D. A. Filatov, 2007. Widespread positive selection in the photosynthetic Rubisco enzyme. BMC Evol. Biol. 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte, V., and B. Charlesworth, 2002. Effective population size and population subdivision in demographically structured populations. Genetics 162: 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte, V., D. A. Filatov, E. Kamau and D. Charlesworth, 2005. Indirect evidence from DNA sequence diversity for genetic degeneration of the Y-chromosome in dioecious species of the plant Silene: the SlY4/SlX4 and DD44-X/DD44-Y gene pairs. J. Evol. Biol. 18: 337–347. [DOI] [PubMed] [Google Scholar]
- Mastenbroek, O., and J. van Brederode, 1986. The possible evolution of Silene pratensis as deduced from present day variation patterns. Biochem. Syst. Ecol. 14: 165–181. [Google Scholar]
- Mastenbroek, O., P. Hogeweg, J. Heringa, G. J. Niemann and J. van Nigtevecht, 1984. Isozyme variation in Silene pratensis: a response to different environments. Biochem. Syst. Ecol. 12: 29–36. [Google Scholar]
- McCauley, D. E., 1994. Contrasting the distribution of chloroplast DNA and allozyme polymorphism among local populations of Silene alba: implications for studies of gene flow in plants. Proc. Natl. Acad. Sci. USA 91: 8127–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley, D. E., 1997. The relative contributions of seed and pollen movement to the local genetic structure of Silene alba. J. Hered. 88: 257–263. [Google Scholar]
- Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
- Prentice, H. C., 1976. A study in endemism: Silene diclinis. Biol. Conserv. 10: 15–30. [Google Scholar]
- Prentice, H. C., 1978. Experimental taxonomy of Silene section Elisanthe (Caryophyllaceae): crossing experiments. Bot. J. Linn. Soc. 77: 203–216. [Google Scholar]
- Prentice, H. C., 1984. Enzyme polymorphism, morphometric variation and population structure in a restricted endemic, Silene diclinis (Caryophyllaceae). Biol. J. Linn. Soc. 22: 125–143. [Google Scholar]
- Przeworski, M., 2002. The signature of positive selection at randomly chosen loci. Genetics 160: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, A. R., 1995. Genetic evidence for a Pleistocene population explosion. Evolution 49: 608–615. [DOI] [PubMed] [Google Scholar]
- Rogers, A. R., and H. Harpending, 1992. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 9: 552–569. [DOI] [PubMed] [Google Scholar]
- Rozas, J., J. C. Sánchez-DelBarrio, X. Messeguer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496–2497. [DOI] [PubMed] [Google Scholar]
- Schneider, S., and L. Excoffier, 1999. Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics. 152: 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen, O., 2004. Hybridization and adaptive radiation. Trends Ecol. Evol. 19: 198–207. [DOI] [PubMed] [Google Scholar]
- Slatkin, M., 1976. The rate of spread of an advantageous allele in a subdivided population, pp. 767–780 in Population Genetics and Ecology, edited by S. Karlin and E. Nevo. Academic Press, New York.
- Slatkin, M., and R. R. Hudson, 1991. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129: 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin, M., and L. Voelm, 1991. FST in a hierarchical island model. Genetics 127: 627–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer, R. J., and M. E. Salvucci, 2002. RUBISCO: structure, regulatory interactions, and possibilities for a better enzyme. Ann. Rev. Plant. Biol. 53: 449–475. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, D. R., and S. R. Keller, 2007. Historical range expansion determines the phylogenetic diversity introduced during contemporary species invasion. Evolution 61: 334–345. [DOI] [PubMed] [Google Scholar]
- Tsitrone, A., M. Kirkpatrick and D. A. Levin, 2003. A model for chloroplast capture. Evolution 57: 1776–1782. [DOI] [PubMed] [Google Scholar]
- Vigouroux, V., and D. Couvet, 2000. The hierarchical island model revisited. Genet. Sel. Evol. 32: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeley, J., 1999. Nonequilibrium migration in human history. Genetics 153: 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson, G. A., 1975. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 7: 256–275. [DOI] [PubMed] [Google Scholar]
- Wolfe, K. H., W. H. Li, and P. M. Sharp, 1987. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast and nuclear DNA. Proc. Natl. Acad. Sci. USA 84: 9054–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S., 1931. Evolution in Mendelian populations. Genetics 16: 97–159. [DOI] [PMC free article] [PubMed] [Google Scholar]