Abstract
Cytoplasmic incompatibility (CI) is the most widespread reproductive modification induced in insects by the maternally inherited intracellular bacteria, Wolbachia. Expression of CI in Drosophila melanogaster is quite variable. Published papers typically show that CI expression is weak and often varies between different Drosophila lines and different labs reporting the results. The basis for this variability is not well understood but is often considered to be due to unspecified host genotype interactions with Wolbachia. Here, we show that male development time can greatly influence CI expression in D. melanogaster. In a given family, males that develop fastest express very strong CI. The “younger brothers” of these males (males that take longer to undergo larval development) quickly lose their ability to express the CI phenotype as a function of development time. This effect is independent of male age effects and is enhanced when flies are reared under crowded conditions. No correlation is seen between this effect and Wolbachia densities in testes, suggesting that a more subtle interaction between host and symbiont is responsible. The observed younger brother effect may explain much of the reported variability in CI expression in this species. When male development time is controlled, it is possible to obtain consistently high levels of CI expression, which will benefit future studies that wish to use D. melanogaster as a model host to unravel CI mechanisms.
WOLBACHIA are intracellular, maternally inherited, α-Proteobacteria known to infect a broad range of invertebrates, including crustaceans, mites, filarial nematodes, spiders, and at least 25% of all insect species (Werren 1997; Jeyaprakash and Hoy 2000; Floate et al. 2006). In arthropods, Wolbachia commonly act as reproductive parasites and manipulate their host's reproduction in a variety of ways, including male killing, feminization of genetic males, parthenogenesis induction, or more commonly via cytoplasmic incompatibility (CI) (Werren and O'Neill 1997). It is considered that all these phenotypes provide a reproductive advantage to infected females, thereby allowing Wolbachia to persist and spread into host populations (Hoffmann and Turelli 1997).
CI is the best-described reproductive modification caused by Wolbachia. CI results in failure to produce progeny in crosses between an infected male and a female that lacks the same strain of Wolbachia found in the male. The reciprocal cross between uninfected males and infected females is fertile, as are crosses between males and females harboring the same Wolbachia strain. CI provides a reproductive advantage to infected females since they can mate successfully with either infected or uninfected males, while uninfected females are incompatible with infected males (Werren and O'Neill 1997). Therefore, as a consequence of maternal inheritance of Wolbachia-infected individuals will increase in frequency in a host population. Though the molecular mechanisms of CI have not yet been identified, several lines of evidence suggest that the proper functioning of sperm is modified by Wolbachia infection (Werren 1997; Poinsot et al. 2003). Cytological studies demonstrate a delay in nuclear envelope breakdown and a disruption of paternal chromosome condensation in CI embryos during the first mitotic division, leading to their subsequent death (Callaini et al. 1997; Tram and Sullivan 2002; Tram et al. 2003).
Werren proposed a nomenclature for describing Wolbachia strains based on the modification status of male sperm and the ability of female embryos to rescue this modification. In this system, four phenotypic categories can be expected: mod+/resc+, mod+/resc−, mod−/resc+, and mod−/resc- (Werren 1997). In wild Drosophila simulans population, five distinct Wolbachia strains have been reported to date (Mercot and Charlat 2004). wRi, wHa, and wNo have been described as mod+/resc+ strains. These three Wolbachia strains induce distinctive CI phenotypes in D. simulans. The wRi strain induces high CI, while wHa and wNo show partial CI (Mercot and Charlat 2004). In addition, two mod− strains have been described from D. simulans; wMa displays a mod−/resc+ phenotype (Mercot and Poinsot 1998a; Charlat et al. 2003) and wAu is considered a mod−/resc− strain, which induces no CI and does not appear to rescue the modification of all mod+ strains tested so far (James and Ballard 2000; Reynolds and Hoffmann 2002; Charlat et al. 2003, 2004).
In D. melanogaster, Wolbachia infection also induces CI (Hoffmann 1988), but its expression appears much more variable. Early studies have shown CI expression ranging from 0 to 77% incompatible embryos (Holden et al. 1993; Hoffmann et al. 1994; Solignac et al. 1994). Genetic characterization of Wolbachia strains, based on gene sequences such as 16S rDNA (Holden et al. 1993), ftsZ (Werren et al. 1995), dnaA (Bourtzis et al. 1994), and wsp (Zhou et al. 1998), have all concluded that D. melanogaster is predominantly infected by a single mod+/resc+ strain of Wolbachia known as wMel. However, expression of CI by this strain appears to be very variable, with different studies reporting results ranging from very strong to very weak CI (Bourtzis et al. 1996; Poinsot et al. 1998; McGraw et al. 2002; Reynolds and Hoffmann 2002). The basis of this variability is not well understood but is often considered to be due to host genetic background differences. Another possible explanation is that the wMel strain actually consists of different cryptic variants. A recent report has characterized five different Wolbachia genetic variants within stocks of D. melanogaster (Riegler et al. 2005), although phenotypic variation associated with these strains is unclear.
Several environmental and physiological factors have been identified that influence the expression of CI in D. simulans. For example, infected males exposed to nutritional stress have a decreased ability to induce CI (Sinkins et al. 1995; Clancy and Hoffmann 1998). Similarly, males that have multiply mated also show reduced expression of CI (Karr et al. 1998) as do old males (Hoffmann et al. 1990; Turelli and Hoffmann 1995). The influence of these factors on expression of CI in D. melanogaster is unclear.
Only one study has shown very strong CI in wMel-infected D. melanogaster (Reynolds and Hoffmann 2002). In this study, Reynolds and Hoffmann clearly showed the importance of a male age effect in D. melanogaster. They described that CI levels declined rapidly with increasing male age in both wMel- and wMelCS-infected lines. Notably, 1-day-old males showed almost perfect CI, while 5-day-old males expressed no CI. Following the discovery of a male age effect, very young males have been used for CI tests in recent studies (Veneti et al. 2003; Fry et al. 2004). Unexpectedly, Veneti et al. (2003) observed weak CI in 3 different variants (25% with wMel, 0% with wMelCS and wMelPop) and Fry et al. (2004) observed no CI with wMel, despite using young males. Taken together, the large fluctuations of CI levels reported within single host lines under the same experimental conditions (Solignac et al. 1994; Poinsot et al. 1998) suggests that factors other than male age are influencing CI levels in D. melanogaster.
In this study, we show for the first time, to our knowledge, an effect of male development time on CI expression. In D. melanogaster, consistently high levels of CI are expressed when the fastest developing males are used in crosses. The “younger brothers” of these males quickly lose their ability to express the CI phenotype as a function of development time. The observed younger brother effect may explain much of the reported variability in CI expression in D. melanogaster.
MATERIALS AND METHODS
Fly lines:
Fly lines were kept on a standard corn diet at a constant temperature of 25°. The D. melanogaster strain designated BNE used in this work originated from field-caught flies collected in Brisbane, Queensland, Australia in 2004 (BNE) and 2006 (BNE2). BNE and BNE2 are both infected with the wMel Wolbachia strain (Riegler et al. 2005). Virgin females from D. melanogaster yw67c23 infected with the wMel Wolbachia strain were mated to males of the BNE line. Female offspring were then backcrossed to males of the BNE line for a total of five generations. The resulting wMel(BNE) line was maintained for a further five generations before performing CI assays. Cured wMel(BNE) and BNE2 lines were subsequently generated by tetracycline treatment following established protocols (Hoffmann 1988) and designated BNE-T and BNE2-T. The following Wolbachia-infected D. melanogaster and D. simulans lines were used in this work: D. melanogaster Canton-S carrying the wMelCS Wolbachia strain, Harwich carrying the wMel (Riegler et al. 2005), D. simulans N7No carrying the wNo (Mercot and Poinsot 1998b), Coffs Harbour carrying wAu (Hoffmann et al. 1996), and DSH carrying the wHa infection (O'Neill and Karr 1990). Wolbachia genotypes were characterized using polymorphic markers (Riegler et al. 2005; Miller and Riegler 2006). Cured lines were generated and designated CS-T, Hw-T, No-T, Ha-T, and Au-T.
Rearing conditions:
To standardize rearing conditions for CI tests, flies (n = 100 aged ∼3–5 day, male and female mixed population) were grown under controlled low density conditions. One hundred flies (3–5 days old, male and female mixed population) were collected from stock bottles and placed into plastic bottle egging chambers. They were allowed to oviposit for 5 hr, and then 200 eggs were counted and transferred to fresh bottles containing 40 ml of diet. This ensured that all flies used in subsequent crosses had been reared under standardized conditions of low density. All flies were incubated at 25° with a 12-hr-light/dark cycle.
CI tests:
Unless noted, male flies were used in CI tests within 24 hr of eclosing to avoid any complications arising from diminishing incompatibility with increasing male age. Female flies <5 days old were used in crosses. For D. melanogaster, single pairs of males and females were placed in empty vials and visually monitored for mating. Pairs that failed to mate were excluded. The Wolbachia infection status of mated males was confirmed by PCR of the wsp gene using primers 81F and 691R (Braig et al. 1998). Females were transferred to plastic bottles with molasses plate lids that were dotted with yeast suspension. Eggs were collected at 25° every 24 hr on molasses plates over a period of 3 days. Females that laid <30 eggs in the total of three plates were discarded from the experiment. The plates were placed at 25° for a further 36–48 hr and the number of total and unhatched eggs was counted. Statistical significance of hatch rates for various crosses was determined using a Mann-Whitney U-test. For D. simulans, single pairs of virgin males and females were introduced to plastic bottles with molasses plate lids. They were given 24 hr to mate, then the males were removed and the females were allowed to lay eggs. The same procedure as above was followed to collect eggs.
Wolbachia density measurement:
Virgin male flies were collected within 7 hr of eclosion and incubated overnight in standard food vials and then frozen at −80°. DNA of single flies was extracted using the Holmes-Bonner method (Holmes and Bonner 1973). DNA of testes was extracted using the simplified STE method (O'Neill et al. 1992). In brief, a single pair of testes was dissected into 20 μl of STE (100 mm NaCl/10 mm Tris-HCl, pH 8.0/1 mm EDTA, pH 8.0) containing 1 mg/ml proteinase K and incubated for 30 min at 37° followed by 5 min at 95°. Samples were vortexed and briefly centrifuged, and 1 μl of the supernatant was used as the template in subsequent quantitative PCR (Q-PCR) using the LightCycler system (Roche) with SYBR Green (Invitrogen, Carlsbad, CA). Primers were designed to amplify 69-bp regions of the single copy Wolbachia WD1063/wsp gene (444F 5′-AGCGTATATTAGCACTCCTTTGGAA and 512R 5′- TGACCAGCAAAACCAAATTTACTTT). A temperature profile of 95° for 5 sec, 60° for 5 sec, and 72° for 10 sec was used for 50 cycles. Initial copy number was estimated by comparison to a standard curve using Roche LightCycler data analysis software v3.1.02. Three replicates were run and averaged for each sample. For each eclosion-day point, we collected measurement on five samples. Statistical analysis was performed using Mann-Whitney U-test.
Immunological studies:
Testes were dissected in TBST (25 mm Tris, 137 mm NaCl, 5 mm KCl, 0.1% Tween, pH 7.5) and fixed with TBST containing 3.7% formaldehyde for 30 min. After washing with TBST, testes were incubated overnight at 4° in a 1:500 dilution of anti-WSP polyclonal antibody (Dobson et al. 1999) in TBST with 1% BSA. After removing the primary antibody with TBST, testes were incubated for 1 hr at room temperature in a 1:500 dilution of Alexa Fluor 488 goat anti-rabbit IgG (H+L) antibody (Molecular Probes, Eugene, OR; no. A11034) in TBST with 1% BSA. Testes were then washed in TBST and stained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 5 min, washed again, and mounted with 80% glycerol. Individual cysts were removed from the testes and stained on poly-l-lysine-coated slides according to Clark et al. (2002).
RESULTS
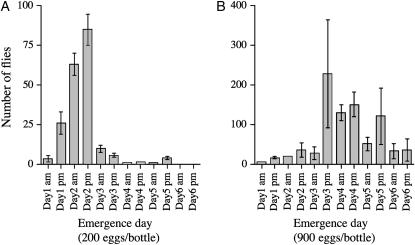
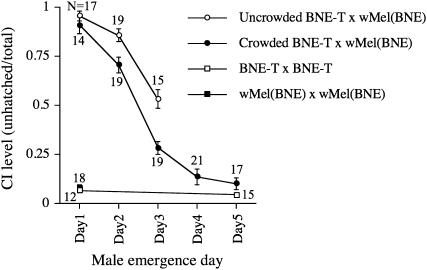
The complete genome sequence of the Wolbachia wMel strain was determined using Wolbachia isolated from the D. melanogaster yw67c23 line (Wu et al. 2004). However, yw67c23 males failed to copulate within a 1-hr observation period in our lab, presumably due to mutations in the yellow and white genes (Burnet et al. 1973; Sciandra and Bennett 1976; R. Yamada, unpublished results), while wild-type D. melanogaster males typically copulate within 5 min. To facilitate standardized CI test conditions using visually monitored mating, we generated a field-caught wild-type D. melanogaster line carrying wMel originating from yw67c23, designated wMel(BNE). We tested if male development time influenced the strength of CI expression in D. melanogaster using wMel(BNE) under controlled uncrowded conditions (200 eggs seeded per bottle). Adult fly emergence under these conditions was very synchronous, occurring predominantly within a 2-day time interval (Figure 1A). In crosses between day 1-eclosing infected males and uninfected BNE-T females, the CI level (mean unhatched eggs) was 0.95 (n = 17) compared to 0.02 (n = 12) in crosses where both sexes were uninfected (Figure 2). The high CI induced by day 1 males declined with increasing larval development time. Day 2 males induced slightly weaker CI (0.85, n = 19) in comparison to that of the Day 1 males (Mann-Whitney, P = 0.01), while day 3 males induced greatly reduced CI (0.529, n = 15) when compared to day 1 males (Mann-Whitney, P < 0.0001).
Figure 1.—
Distribution of fly emergence in uncrowded conditions with 200 eggs per bottle (A) and crowded conditions with 900 eggs per bottle (B). wMel(BNE) flies were kept on a 12-hr-light/dark cycle and emerging flies were counted every morning (9:00 am) and evening (5:00 pm). Bars, mean number of flies emerging per bottle (n = 3). Error bars indicate SEM.
Figure 2.—
Younger brother effect on the level of CI in wMel-infected D. melanogaster. Crosses were performed as follows: open circles, BNE-T females × wMel(BNE) males grown under uncrowded conditions of 200 eggs per bottle; closed circles, BNE-T females × wMel(BNE) males grown under crowded conditions of 900 eggs per bottle; open square, BNE-T females × BNE-T males compatible cross control; closed square, wMel(BNE) females × wMel(BNE) males compatible cross control. Values beside circles and squares represent number of single-female replicates. Error bars indicate SEM.
Under crowded conditions (900 eggs seeded per bottle), flies eclosed over a longer time period with greater variability (Figure 1B). When males from these bottles were used in CI test crosses, it was found that initial CI strength from day 1 males was indistinguishable from equivalent males from uncrowded bottles. However, males collected on subsequent days showed significantly reduced CI expression compared to equivalent males reared under uncrowded conditions (Figure 2). Males eclosing on day 5 expressed no CI, hatch rates being indistinguishable from control crosses.
The expression of very strong CI in the earliest eclosing males could be a function of the development time of the males used in test crosses. Alternatively, it might be due to effects associated with mothers of these males. In particular, the age at which females lay eggs might influence Wolbachia levels and development time of the males that were subsequently used in test crosses. To control for these possible effects, 50 wMel(BNE) virgin females were aged for 3 days in bottles and then mixed with 50 wMel(BNE) virgin males for 24 hr to mate. Consequently, flies were transferred to plastic bottles to lay eggs for 1 hr (early eggs). After egg collection, flies were transferred to fresh food bottles every day and allowed to lay eggs continuously. After 7 days, flies were transferred to plastic bottles again to collect eggs (late eggs). CI tests were performed with males that had developed from early or late eggs. There was no significant difference between these males (data not shown), suggesting that the loss of CI strength is independent of the age of female parents of the males and depends solely on development time of individual males. The males that develop fastest express strongest CI, while the younger brothers of these males lose their ability to induce CI as a function of development time.
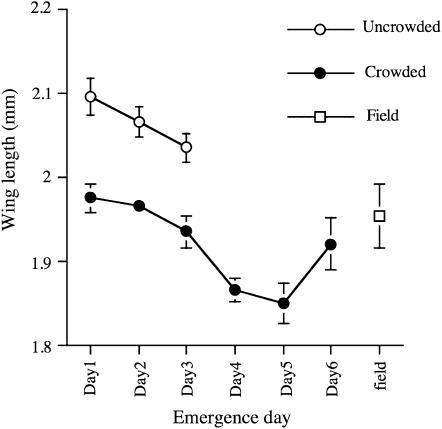
Development time is known to correlate with body size and was examined as a possible explanation for the observed effect. To compare body size, we measured the wing length of male flies with differing development times and rearing densities (Figure 3). Late eclosing males had shorter wing lengths than early eclosing males in both crowded and uncrowded conditions. However, there was no direct correlation between body size and CI strength. For example, uncrowded day 3 males were larger than crowded day 1 males, but CI data showed that the smaller flies eclosing earlier expressed stronger CI than the late eclosing larger flies. Therefore, the younger brother effect cannot be explained simply as a consequence of male size.
Figure 3.—
Mean wing length of male flies. Open circles, wMel(BNE) males grown under uncrowded conditions of 200 eggs per bottle. Closed circles, wMel(BNE) males grown under crowded conditions of 900 eggs per bottle. Open square, field-collected (Brisbane) male D. melanogaster. Error bars indicate SEM (n = 10).
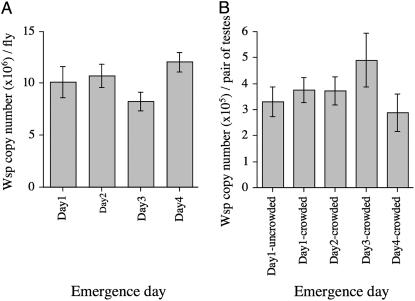
It is well known that CI strength is dependent on Wolbachia densities in insects (Breeuwer and Werren 1993; Bressac and Rousset 1993; Bourtzis et al. 1996; Bordenstein et al. 2006) and it is possible that the observed effect could be explained by a relationship between male development time and Wolbachia density. We examined Wolbachia densities in flies collected on different emergence days by quantitative PCR (Q-PCR) using the single copy wsp gene. Initially wsp gene copy number was quantified from whole flies and no reduction was observed in absolute quantities of Wolbachia despite the smaller size of late eclosing males (Figure 4A). We then examined wsp copy number in dissected testes of males grown under both crowded and uncrowded conditions and again no correlation was found between Wolbachia density and male development time (Figure 4B), even under the more extreme crowded conditions. It is possible that Wolbachia distribution within sperm cysts, rather than density per se, might be a better predictor of CI levels (Clark et al. 2002, 2003; Veneti et al. 2003). Therefore, we performed DAPI staining and immunostaining to visualize Wolbachia density and distribution in testes and cysts. Although day1-eclosed males induce almost perfect CI expression, we could not detect any difference in Wolbachia densities or distribution in cysts between day 1 and day 5 males (data not shown) consistent with the Q-PCR data. As such, the younger brother effect cannot be explained by an interaction between development time and Wolbachia densities in flies.
Figure 4.—
Mean Wolbachia density as determined by quantitative PCR. (A) Mean Wolbachia density for wMel(BNE) adult males eclosing over different days and grown under crowded conditions of 900 eggs per bottle. (B) Mean Wolbachia density for wMel(BNE) testes taken from males eclosing over different days and grown under crowded conditions of 900 eggs per bottle and uncrowded conditions with 200 eggs per bottle. Error bars indicate SEM (n = 5).
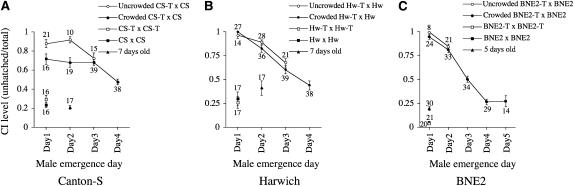
We examined the generality of the younger brother effect by examining different D. melanogaster lines. Canton-S and Harwich are long-established inbred laboratory strains naturally infected with wMelCS and wMel, respectively (Riegler et al. 2005). In wMelCS-infected Canton-S, we observed a weaker younger brother effect (Figure 5A). Under uncrowded conditions, day 2 males induced a similar level of CI (0.92, n = 10) compared to day 1 males (0.88, n = 21), while day 3 males induced reduced CI (0.72, n = 15) when compared to day 1 males (Mann-Whitney, P = 0.03). Under crowded condition, the initial CI strength from day 1 males (0.71, n = 16) was distinguishable from equivalent males from uncrowded bottles (Mann-Whitney, P = 0.02), whereas day 2 and day 3 males induced similar CI strength (0.68, n = 19; 0.679, n = 39, respectively) when compared to day 1 males. The CI strength declined in day 4 males (0.47, n = 38) compared to day 3 males under crowded conditions (Mann-Whitney, P < 0.001). In wMel-infected Harwich, the younger brother effect was obvious in both uncrowded and crowded conditions (Figure 5B). In addition, the recently field-caught BNE2 line infected with wMel was also examined for the effect. In this line, a strong younger brother effect was observed in both uncrowded and crowded conditions (Figure 5C). To examine the influence of male age effects, day 1 males were aged for 5 days and then used for CI tests. Five-day-old males that had originally eclosed on day 1 showed a reduced ability to express CI (0.19, n = 30) when compared to 1-day-old males (Mann-Whitney, P < 0.001) (Figure 5C). Seven-day-old males expressed no CI in the Canton-S and Harwich lines, although equivalent males induced high CI if they were only 1 day old (Figure 5, A and B).
Figure 5.—
Younger brother effect on the level of CI in different D. melanogaster lines. (A) Canton-S line. (B) Harwich line. (C) BNE2 line. Crosses were performed as follows: open circles, uninfected females × infected males grown under uncrowded conditions of 200 eggs per bottle; closed circles, uninfected females × infected males grown under crowded conditions of 900 eggs per bottle; open square, uninfected females × uninfected males compatible cross control; closed square, infected females × infected males compatible crosses control; closed triangle, uninfected females × 7-day-old infected males (A and B) and uninfected females × 5-day-old infected males (C). Values beside circles and squares represent number of single-female replicates. Error bars indicate SEM.
We also examined Wolbachia strains in D. simulans. As observed for wMel in D. melanogaster, CI levels associated with the wNo strain in D. simulans have been reported as quite variable, making it a potential candidate for the younger brother effect (Mercot et al. 1995; Mercot and Poinsot 1998a; James et al. 2002; Veneti et al. 2003; Mercot and Charlat 2004). Under our experimental conditions, wNo induced strong CI expression. In crosses between day 1 wNo-infected males and uninfected females, mean egg hatch failure was 0.86 (n = 21) compared to 0.09 (n = 21) in crosses where both sexes were uninfected (Table 1). While there was some variability in CI levels between males with different development time, there was no clear younger brother effect. We also examined wHa- and wAu-infected D. simulans. The wHa infection expressed strong CI regardless of male development time. The wAu line expressed no CI even when day 1 males where used in the test cross (Table 1). This is consistent with the previous report that the wAu strain does not induce CI (James and Ballard 2000; Reynolds and Hoffmann 2002; Charlat et al. 2003, 2004).
TABLE 1.
Effect of male development time on CI in Wolbachia-infected D. simulans
| Female | Male | No. of crosses | Total eggs counter | Mean CI (unhatched/total) | SE |
|---|---|---|---|---|---|
| No-T | wNo-Day1 | 21 | 1387 | 0.862 | 0.022 |
| No-T | wNo-Day2 | 23 | 2262 | 0.779 | 0.025 |
| No-T | wNo-Day3 | 22 | 2361 | 0.798 | 0.042 |
| No-T | wNo-Day4 | 25 | 2770 | 0.835 | 0.031 |
| No-T | No-T | 21 | 1530 | 0.089 | 0.011 |
| Ha-T | wHa-Day1 | 9 | 734 | 0.994 | 0.006 |
| Ha-T | wHa-Day2 | 10 | 726 | 0.987 | 0.007 |
| Ha-T | wHa-Day3 | 3 | 243 | 0.870 | 0.065 |
| Ha-T | Ha-T | 10 | 920 | 0.096 | 0.009 |
| Au-T | wAu-Day1 | 19 | 2118 | 0.346 | 0.058 |
| wAu | wAu-Day | 20 | 2028 | 0.363 | 0.045 |
| Au-T | Au-T | 10 | 928 | 0.302 | 0.092 |
DISCUSSION
In studies of the Wolbachia symbiosis of insects during the last 20 years, D. simulans has been used more heavily as a model host than D. melanogaster, despite the wealth of genetic tools available in the latter. One of the reasons for this is the highly variable expression of the CI phenotype in D. melanogaster, making its study difficult. In different papers, a variety of CI levels have been reported, varying from 0 to almost 100%. Recent work from Reynolds and Hoffmann (2002) indicated that almost 100% CI could be obtained with wMel-infected 1-day-old males but not with older males, indicating a strong male age effect in this species. However, even when this factor has been controlled in subsequent experiments, variability in CI expression has still been seen. For example, Fry et al. (2004) observed no CI expression in wMel-infected lines, even though they used ∼16- to 40-hr posteclosion males. In a study reported by Veneti et al. (2003), 1-day-old males were used in CI crosses; however, wMel-infected lines showed low CI levels (25%), while wMelCS and wMelPop showed no CI. Similarly, McGraw et al. (2002) detected weak CI using wMel-infected 1- to 2-day-old males.
One possible confounding factor in these different studies is the possibility of multiple matings by males. Reynolds and Hoffmann (2002) separated males and females after mating to avoid repeated copulation. In contrast, other studies have left males and females together during the egg collection period. Repeated copulation is a factor that is known to reduce CI in D. simulans (Karr et al. 1998). If males remained with females for several days, CI expression could be diminished by both repeated copulation and subsequent matings with older males. In contrast, isolated females produce constant CI levels for 5 days after copulation (R. Yamada, unpublished results). However, in the report of Fry et al. (2004), eggs were collected from females kept with males for one 24-hr period, limiting the possibility of either male age or multiple mating effects. CI levels would have been predicted under these conditions to be around 50%, but no CI was found.
Although some of these contradictions might have been due to repeated copulation and/or host line differences, much of it might be due to an undescribed factor influencing CI levels, independently of male age effects. In this study, we found that a rapid decline in CI levels is correlated with male development time. This effect is independent of male age. For example, 1-day-old males expressed high CI levels if they had undergone fast development, whereas no CI was detected with 1-day-old males that had undergone slow development. However, males that develop fastest lose their ability to express CI as they age. These results suggest that male development time and male age influence CI expression independently. Male development time influenced CI expression in all D. melanogaster lines examined, including North American inbred lab lines (Canton-S, Harwich) and a recently caught Australian (BNE2) wild-type strain as well as across two Wolbachia genotypes (wMel and wMelCS), indicating that this is a general effect in D. melanogaster. We refer to this observation as the younger brother effect, and it may explain much of the reported variability in CI expression in D. melanogaster.
We examined three possibilities to explain the relationship between CI levels and observed development time differences. First, slower developing males might lose the infection, resulting in the presence of uninfected males in CI crosses. This can be excluded by the observation that the Wolbachia infection in males was present in nearly 100% of individuals across all development times. The infection status of males was confirmed by PCR after copulation and data from PCR-negative males were excluded in our analysis. Second, fastest developing males might have originated from eggs laid earlier in the life of females, which in turn may have influenced CI. Larvae from these eggs may develop faster and contain higher levels of Wolbachia. If a female effect such as this existed, then males that develop from eggs laid by older females should express lower CI than males that develop from eggs laid by younger females. No difference in CI levels of sons derived from either young or old females was observed.
Third, a relationship might exist between Wolbachia density and development time such that highly infected larvae develop faster than larvae infected at low levels. There are a number of reports suggesting a positive correlation between Wolbachia density and strength of CI in many insect species, including Drosophila. In an earlier study, Bressac and Rousset (1993) found a decrease in the frequency of infected sperm cysts with age, which might correlate with the reduction of CI levels in older males. Following this discovery, Clark et al. (2002) found that fewer cysts are infected in wMel-infected D. melanogaster than wRi-infected D. simulans. Recently, Veneti et al. (2003) showed a relationship between the percentage of infected cysts and CI levels in a variety of Wolbachia strains. In their report, mod+ strains, including wRi, wHa, wNo, and wMel showed a positive correlation between infected cysts and CI levels. On the basis of this hypothesis, testes of fast developing males should carry higher infection densities than slower developing males. However, we failed to detect any difference in either Wolbachia density in testes or frequency of infected cysts between fast and slow developing males. In our data, all wMel-infected males showed a low infection frequency of cysts (<10%). This observation is consistent with previous studies (Veneti et al. 2003). It is possible that Wolbachia are lost after eclosion, although sperm chromosomes are fully modified in the early stages of development. The loss of Wolbachia-infected cysts happens around day 3 posteclosion in D. simulans (Clark et al. 2002). It is possible that slower developing males lose their Wolbachia, whereas fast developing males maintain a Wolbachia density necessary for high CI induction. To test this, we examined the infection status of newly eclosed males prior to the standard time allowed for maturation before being used in crossing studies. Again, we saw no difference between fast and slow developing males by both Q-PCR of testes and DAPI staining of sperm cysts (data not shown).
The effect of crowding on expression of the younger brother effect was quite pronounced, suggesting that nutritional stress may play a role in its expression. However, neither nutritional condition nor body size per se is known to directly influence CI strength (Clancy and Hoffmann 1998; our data). While the younger brother effect appears to be quite strong in D. melanogaster, the mechanism by which it acts seems independent of bacterial density. It also seems to be largely absent in D. simulans. Understanding this effect and controlling for it in experiments allows consistently high levels of CI to be expressed, which will greatly facilitate the use of D. melanogaster as a model organism to determine the molecular mechanisms by which Wolbachia is so successfully able to manipulate the reproduction of its host.
Acknowledgments
We thank Manpreet Sidhu and Jenny Gough for technical support and assistance and Peter Cook, David Merritt, and Jason Rice for help with experiments. We also thank Michael Clark, Elizabeth McGraw, and Wolfgang Miller for helpful discussion and Iñaki Iturbe-Ormaetxe and Jeremy Brownlie for providing constructive comments on the manuscript. This work was supported by grants from the Australian Research Council.
References
- Bordenstein, S. R., M. L. Marshall, A. J. Fry, U. Kim and J. J. Wernegreen, 2006. The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathog. 2: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis, K., A. Nirgianaki, P. Onyango and C. Savakis, 1994. A prokaryotic dnaA sequence in Drosophila melanogaster: Wolbachia infection and cytoplasmic incompatibility among laboratory strains. Insect Mol. Biol. 3: 131–142. [DOI] [PubMed] [Google Scholar]
- Bourtzis, K., A. Nirgianaki, G. Markakis and C. Savakis, 1996. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144: 1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig, H. R., W. Zhou, S. L. Dobson and S. L. O'Neill, 1998. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 180: 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer, J. A., and J. H. Werren, 1993. Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressac, C., and F. Rousset, 1993. The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J. Invertebr. Pathol. 61: 226–230. [DOI] [PubMed] [Google Scholar]
- Burnet, B., K. Connolly and B. Harrison, 1973. Phenocopies of pigmentary and behavioral effects of the yellow mutant in Drosophila induced by alpha-dimethyltyrosine. Science 181: 1059–1060. [DOI] [PubMed] [Google Scholar]
- Callaini, G., R. Dallai and M. G. Riparbelli, 1997. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J. Cell Sci. 110(Pt. 2): 271–280. [DOI] [PubMed] [Google Scholar]
- Charlat, S., L. Le Chat and H. Mercot, 2003. Characterization of non-cytoplasmic incompatibility inducing Wolbachia in two continental African populations of Drosophila simulans. Heredity 90: 49–55. [DOI] [PubMed] [Google Scholar]
- Charlat, S., M. Riegler, I. Baures, D. Poinsot, C. Stauffer et al., 2004. Incipient evolution of Wolbachia compatibility types. Evolution Int. J. Org. Evolution 58: 1901–1908. [DOI] [PubMed] [Google Scholar]
- Clancy, D. J., and A. A. Hoffmann, 1998. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol. Exp. Appl. 86: 13–24. [Google Scholar]
- Clark, M. E., Z. Veneti, K. Bourtzis and T. L. Karr, 2002. The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila. Mech. Dev. 111: 3–15. [DOI] [PubMed] [Google Scholar]
- Clark, M. E., Z. Veneti, K. Bourtzis and T. L. Karr, 2003. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech. Dev. 120: 185–198. [DOI] [PubMed] [Google Scholar]
- Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou et al., 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29: 153–160. [DOI] [PubMed] [Google Scholar]
- Floate, K. D., G. K. Kyei-Poku and P. C. Coghlin, 2006. Overview and relevance of Wolbachia bacteria in biocontrol research. Biocontrol Sci. Tech. 16: 767–788. [Google Scholar]
- Fry, A. J., M. R. Palmer and D. M. Rand, 2004. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93: 379–389. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A., 1988. Partial cytoplasmic incompatibility between two Australian populations of Drosophila melanogaster. Entomol. Exp. Appl. 48: 61–67. [Google Scholar]
- Hoffmann, A. A., and M. Turelli, 1997. Cytoplasmic incompatibility in insects, pp. 42–80 in Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, edited by S. L. O'Neill, A. A. Hoffmann and J. H. Werren. Oxford University Press, Oxford.
- Hoffmann, A. A., M. Turelli and L. G. Harshman, 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126: 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., D. J. Clancy and E. Merton, 1994. Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., D. Clancy and J. Duncan, 1996. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76(Pt. 1): 1–8. [DOI] [PubMed] [Google Scholar]
- Holden, P. R., P. Jones and J. F. Brookfield, 1993. Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet. Res. 62: 23–29. [DOI] [PubMed] [Google Scholar]
- Holmes, D. S., and J. Bonner, 1973. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry 12: 2330–2338. [DOI] [PubMed] [Google Scholar]
- James, A. C., and J. W. Ballard, 2000. Expression of cytoplasmic incompatibility in Drosophila simulans and its impact on infection frequencies and distribution of Wolbachia pipientis. Evolution Int. J. Org. Evolution 54: 1661–1672. [DOI] [PubMed] [Google Scholar]
- James, A. C., M. D. Dean, M. E. McMahon and J. W. Ballard, 2002. Dynamics of double and single Wolbachia infections in Drosophila simulans from New Caledonia. Heredity 88: 182–189. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash, A., and M. A. Hoy, 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9: 393–405. [DOI] [PubMed] [Google Scholar]
- Karr, T. L., W. Yang and M. E. Feder, 1998. Overcoming cytoplasmic incompatibility in Drosophila. Proc. Biol. Sci. 265: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, E. A., D. J. Merritt, J. N. Droller and S. L. O'Neill, 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99: 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercot, H., and S. Charlat, 2004. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica 120: 51–59. [DOI] [PubMed] [Google Scholar]
- Mercot, H., and D. Poinsot, 1998. a … and discovered on Mount Kilimanjaro. Nature 391: 853. [DOI] [PubMed] [Google Scholar]
- Mercot, H., and D. Poinsot, 1998. b Wolbachia transmission in a naturally bi-infected Drosophila simulans strain from New-Caledonia. Entomol. Exp. Appl. 86: 97–103. [Google Scholar]
- Mercot, H., B. Llorente, M. Jacques, A. Atlan and C. Montchamp-Moreau, 1995. Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics 141: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. J., and M. Riegler, 2006. Evolutionary dynamics of wAu-like Wolbachia variants in neotropical Drosophila spp. Appl. Environ. Microbiol. 72: 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, S. L., and T. L. Karr, 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348: 178–180. [DOI] [PubMed] [Google Scholar]
- O'Neill, S. L., R. Giordano, A. M. Colbert, T. L. Karr and H. M. Robertson, 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89: 2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsot, D., K. Bourtzis, G. Markakis, C. Savakis and H. Mercot, 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsot, D., S. Charlat and H. Mercot, 2003. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: confronting the models with the facts. BioEssays 25: 259–265. [DOI] [PubMed] [Google Scholar]
- Reynolds, K. T., and A. A. Hoffmann, 2002. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. 80: 79–87. [DOI] [PubMed] [Google Scholar]
- Riegler, M., M. Sidhu, W. J. Miller and S. L. O'Neill, 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 15: 1428–1433. [DOI] [PubMed] [Google Scholar]
- Sciandra, Jr., R. J., and J. Bennett, 1976. Behavior and single gene substitution in Drosophila melanogaster. I. Mating and courtship differences with w, cn, and bw loci. Behav. Genet. 6: 205–218. [DOI] [PubMed] [Google Scholar]
- Sinkins, S. P., H. R. Braig and S. L. O'Neill, 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. Biol. Sci. 261: 325–330. [DOI] [PubMed] [Google Scholar]
- Solignac, M., D. Vautrin and F. Rousset, 1994. Widespread occurrence of the proteobacteria Wolbachia and partial cytoplasmic incompatibility in Drosophila melanogaster. C. R. Acad. Sci. III, Sci. Vie 317: 461–470. [Google Scholar]
- Tram, U., and W. Sullivan, 2002. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296: 1124–1126. [DOI] [PubMed] [Google Scholar]
- Tram, U., P. M. Ferree and W. Sullivan, 2003. Identification of Wolbachia host interacting factors through cytological analysis. Microbes Infect. 5: 999–1011. [DOI] [PubMed] [Google Scholar]
- Turelli, M., and A. A. Hoffmann, 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti, Z., M. E. Clark, S. Zabalou, T. L. Karr, C. Savakis et al., 2003. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics 164: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H., 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42: 587–609. [DOI] [PubMed] [Google Scholar]
- Werren, J. H., and S. L. O'Neill, 1997. The evolution of heritable symbionts, pp. 1–41 in Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, edited by S. L. O'Neill, A. A. Hoffmann and J. H. Werren. Oxford University Press, Oxford.
- Werren, J. H., W. Zhang and L. R. Guo, 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. Biol. Sci. 261: 55–63. [DOI] [PubMed] [Google Scholar]
- Wu, M., L. V. Sun, J. Vamathevan, M. Riegler, R. Deboy et al., 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W., F. Rousset and S. O'Neil, 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]