Abstract
Agonists of kainate receptors (KARs) cause both the opening of the associated ion channels and the activation of signalling pathways driven by G-proteins and PKC. Here we report the existence of an unknown mechanism of KAR autoregulation, involving the interplay of this two signalling mechanisms. Repetitive activation of native KARs evoked the rundown of the ionotropic responses in a manner that was dependent on the activation of PKC. Experiments on recombinant GluR5 expressed in neuroblastoma cells indicated that KARs trigger the activation of PKC and induce the internalization of membrane receptors. This phenomenon depends on the PKC-mediated phosphorylation of serines 879 and 885 of the GluR5-2b subunits, since mutation of these two residues abolished internalization. These results reveal that the non-canonical signalling of KARs is associated with a sensitive mechanism that detects afferent activity. Such a mechanism represents an active way to limit overactivation of the KAR system, by regulating the number of KARs in the cell membrane.
Keywords: dorsal root ganglia cells, endocytosis, GluR5, glutamate receptors, trafficking
Introduction
Members of the glutamate receptor subfamily, the so-called kainate receptors (KARs), are widely expressed in the central and peripheral nervous systems (Wisden and Seeburg, 1993; Bahn et al, 1994), where they mediate a number of physiological processes. In the hippocampus and other cortical areas, KARs regulate synaptic transmission by both influencing the release of glutamate and GABA, and by regulating excitability of postsynaptic membrane (reviewed in Lerma, 2003). The role of KARs seems to be mediated by two different signalling pathways (Lerma, 2006). One involves canonical cation channel activity and mediates slow depolarizing synaptic responses (Castillo et al, 1997), which is more similar to the responses mediated by NMDA receptors than those triggered by activation of AMPA receptors. The other represents a non-canonical signalling pathway that involves the activation of a pertussis toxin-sensitive G-protein, resulting in PKC activation (Rozas et al, 2003). While ion channel activity of KARs is unequivocally responsible for part of the excitatory drive of several types of neurons (Castillo et al, 1997; Cossart et al, 1998; Frerking et al, 1998; Kidd and Isaac, 1999; Bureau et al, 2000), the role of the non-canonical (metabotropic) signals triggered by these receptors is less clear. Metabotropic KAR activity has been implicated in regulating the release of both GABA and glutamate (Clark et al, 1997; Rodriguez-Moreno et al, 1997; Rodriguez-Moreno and Lerma, 1998; Frerking et al, 2001), as well as in the excitability of hippocampal pyramidal neurons through the regulation of Ca2+-dependent K+ conductance (Melyan et al, 2002).
Recent data indicate that like AMPA receptor-mediated synaptic responses, those mediated by KARs are subject to long-term synaptic plasticity. For instance, long-term potentiation (LTP)-inducing protocols result in AMPA receptor-mediated LTP at thalamacortical synapses and a reduction in the KAR-mediated component of synaptic responses (Kidd and Isaac, 1999). KAR-mediated synaptic transmission undergoes NMDA-independent long-term depression (LTD) in the perirhinal cortical neurons, which appears to involve the activity of PKC and other effectors (Park et al, 2006). Therefore, it is likely that the synaptic expression of KARs is tightly regulated, although the mechanisms involved remain poorly defined. Interestingly, an attempt to better define these mechanisms demonstrated that GluR6-containing KARs could be endocytosed in a PKC-dependent manner in hippocampal cultured neurons (Martin and Henley, 2004).
All five KAR subunits (GluR5–7, KA1–2) are expressed in the central nervous system. However, peripheral neurons such as primary sensory neurons present in the dorsal root ganglia (DRG) exclusively express GluR5 and KA2 subunits (Bettler et al, 1990; Bahn et al, 1994), and they mostly lack AMPA and NMDA receptor-mediated responses. Both canonical and non-canonical KAR signalling are observed in DRG neurons, which can be readily studied in culture. Indeed, in these cells KARs trigger the activation of a G-protein and of PKC that modulates voltage-dependent Ca2+ channels (Rozas et al, 2003). To further understand how native KARs are regulated, we have studied them in DRG neurons, to test how KARs might be autoregulated by non-canonical signalling. We found that KAR activity stimulates the phosphorylation of S879 and/or S885 in GluR5-2b subunits by PKC, inducing the retrieval of KARs from the membrane. The depression of KAR-mediated membrane currents bidirectionally depends on the intracellular Ca2+ levels, as the effect of PKC is counteracted by calcineurin. Our results identify an unsuspected mechanism by which KARs may detect the level of their activation and limit neuronal excitation by controlling the number of surface receptors.
Results
Rundown of kainate-induced responses in DRG neurons
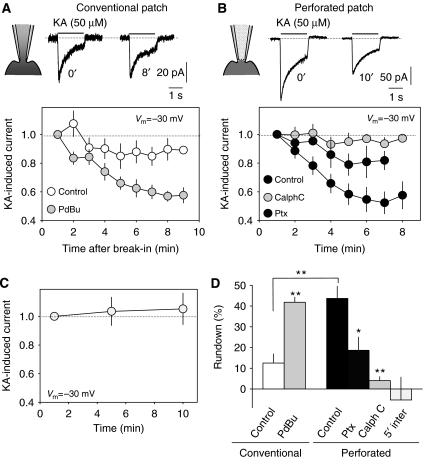
In cultured DRG neurons, whole-cell responses were repeatedly (1 per min) induced by the rapid application of kainate (50 μM, 1.5 s), resulting in a slight time-dependent decline in amplitude (12.5±4.4% in 9 min, n=14; Figure 1A). We will refer to this time-dependent decline as the rundown of KAR-mediated responses. Due to the time required for KARs to recover from desensitization in DRG neurons, this phenomenon could be tested at a maximum of one pulse every minute (not shown). Interestingly, the rundown was significantly larger in neurons recorded under perforated patch conditions, a situation that preserves the internal milieu and processes requiring cell integrity (47.7±7.1% in 7 min, n=12; Figure 1B). Since KAR activity in these cells results in both ion channel opening and the non-canonical activation of PKC (Rozas et al, 2003), we reasoned that PKC activity might best be preserved under perforated patch configuration, thereby influencing KAR rundown. Indeed, the rundown of KAR responses recorded under conventional patch-clamp whole-cell configuration was exacerbated by treating the cells with the phorbol ester PdBu (1 μM, 3 min), declining by 41.8±2.4% (n=18) in the same time period (Figure 1A). On the other hand, the marked rundown under perforated patch recordings was completely abolished by exposing the cells for 3 min to the specific PKC inhibitor calphostin C (1 μM, 4.07±1.9%, P<0.001; Student's t-test; Figure 1B). Similarly, blocking G-protein activity by pertussis toxin (1 μg/ml, 3 h, 37°C) largely prevented rundown of KAR-mediated currents (18.9±6.4%, n=4). In order to determine whether this rundown was frequency dependent, we applied pulses of kainate at 5-min intervals (perforated patch) and found that at this lower frequent activation, KARs did not present rundown (−5.2±10.9% in 10 min, n=5). These data indicate that KAR currents undergo an activity-dependent decline that depends on the activation of PKC, as summarized in Figure 1D.
Figure 1.
Run-down of ionotropic KAR activity in DRG neurons. (A) Under conventional whole-cell configuration (−30 mV holding potential), pulses of KA (50 μM, 1.5 s) were delivered at 1-min intervals. A slight rundown of KA-induced responses was observed (open symbols). Such a rundown was exacerbated by activating PKC (PdBu, 1 μM, light gray symbols). (B) The rundown of KA-induced responses was larger under perforated patch-clamp conditions (closed symbols). This rundown was prevented by inhibiting PKC (calphostin C, 50 μM, gray symbols). (C) The rundown of KA-induced currents depends on KAR activity. Under perforated whole-cell recording, the rundown of KA-induced responses was not apparent if KA pulses were delivered at 5-min intervals. (D) Summary of the data from all the experiments. *P<0.05, **P<0.01; Student's t-test.
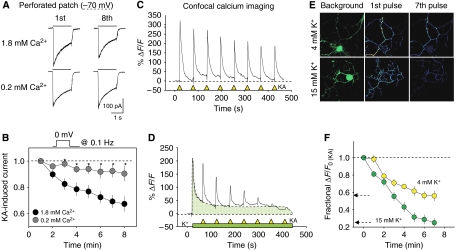
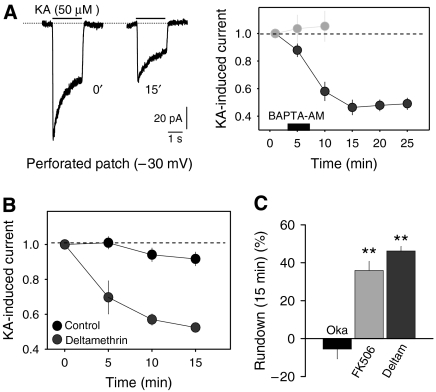
The experiments above were carried out at a holding potential of −30 mV in order to inactivate TTX-resistant Na+ channels that are abundant in the DRG neurons (Fang et al, 2002). Under these conditions, the generation of Na+ spikes is prevented in poorly clamped cell compartments. However, N-type Ca2+ channels are active at these potentials, providing a constant influx of Ca2+ to the cell interior (Hille, 1992; Wakamori et al, 1998), and thus intracellular Ca2+ could participate in this phenomenon. To test the possible role of Ca2+ in KAR rundown, we performed a series of experiments at a constant holding potential of −70 mV (perforated whole-cell configuration) to prevent steady-state Ca2+ channel activity, while controlled influx of Ca2+ was imposed by delivering 100-ms depolarizing pulses to 0 mV at a frequency of 0.1 Hz. Under this experimental regime, KARs were activated every minute with normal (1.8 mM) and low (0.2 mM) concentrations of Ca2+ in the perfusion fluid. Rundown of KAR activity was clearly Ca2+ dependent and it was largely prevented in low extracellular Ca2+ (33±5% in normal Ca2+ versus 7.01±6.8% in low Ca2+, n=8; P<0.05; Figure 2A, B). These same results were observed in calcium imaging experiments where intracellular Ca2+ was steadily elevated by applying a slightly high concentration of K+ (Figure 2C–F). To further study the Ca2+ dependence of KAR rundown, intracellular Ca2+ was chelated by exposing the neurons to BAPTA-AM (5 μM, 5 min). To our surprise, chelating the intracellular Ca2+ exacerbated KAR rundown even at frequencies of KAR activation that did not induce rundown (Figure 3A), attenuating the KAR response by 53.7±5.3% (n=6) in less that 10 min. This may indicate that the recovery of KARs between the exposures to kainate may be regulated by the internal concentration of Ca2+. Therefore, we repeated this experiment, having inhibited the Ca2+/calmodulin-dependent phosphatase calcineurin or PP2B. In the continuous presence of the specific PP2B inhibitor deltamethrin (5 μM) or FK506 (0.5 μM), KAR rundown was evident (46.2±2.4%, n=5, and 35.9±4.8%, n=7, respectively in 15 min; P<0.001; Figure 3C). However, inhibitors of the phosphatase 2A (PP2A), such as okadaic acid (1 μM) had no effect on this process (−5.5±5.2% of initial kainate-induced responses; Figure 3C).
Figure 2.
Low intracellular calcium attenuates activity-dependent rundown of KARs in DRG neurons. (A) Examples of KA-induced responses obtained at the beginning of perforated whole-cell recording and 8 min afterwards at two different extracellular Ca2+ concentrations. To control Ca2+ entry, the holding potential was kept at −70 mV, and 100 ms depolarizing pulses delivered every 10 s. (B) Time course of the decline in KA-induced current decline in normal (1.8 mM) and low Ca2+ (0.2 mM) extracellular concentrations. Points are the average of eight experiments. *P<0.05; Student's t-test. (C–F) Elevating intracellular Ca2+ accelerates rundown of KARs. DRG neurons were loaded with the Ca2+ sensor Fluo4 and imaged by confocal microscopy. Pulses of kainate (KA, 50 μM) were applied every minute (yellow triangles) under normal (4 mM; C) or elevated (15 mM; D) K+. In panel E, top panels show background fluorescence signal (left panels) and the net first and eighth KA-induced Ca2+ responses, obtained after subtracting background fluorescence. Brighter colors indicate larger Ca2+ increases. (F) Data are summarized from seven neurons in each case.
Figure 3.
(A) Chelation of intracellular Ca2+ promotes use-dependent rundown of KAR responses. Ca2+ was chelated by applying BAPTA-AM to DRG neurons for 5 min. Examples of KA-induced responses (perforated whole-cell recording) before and after application of BAPTA-AM to the bath are shown on the left and for comparison, control data (from Figure 1C) has been included in the graph (right, light gray). (B, C) Inhibition of phosphatase 2B—calcineurin—but not PP2A induces rundown of KAR-induced currents at a low activation frequency. Bars in panel C show the average values of 5–7 experiments. The specific PP2B inhibitors deltamethrin and FK506, and that of PP2A, okadaic acid, were used at 5 μM, 0.5 μM and 1 μM, respectively. **P<0.01; Student's t-test.
Taken together, these results indicate that PKC phosphorylates KARs and/or intermediate proteins upon activation of the KARs, provoking a rundown of receptor activity. This is counterbalanced by phosphatase activity, which appears to be mediated at least in part by calcineurin, such that Ca2+ exerts a bidirectional effect on this phenomenon.
PKC-dependent rundown of recombinant KARs
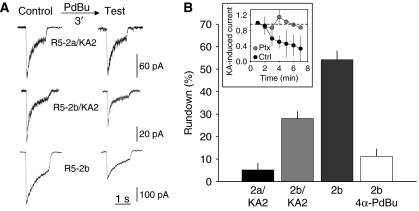
DRG neurons exclusively express GluR5 and KA2 KAR subunits (Bahn et al, 1994). Therefore, we set out to reproduce PKC-induced rundown in cells that express recombinant KAR subunits. We used the undifferentiated SHSY5-Y neuroblastoma cell line that does not express KARs, but that preserves its neuronal character, since it can be easily differentiated to neurons upon treatment with retinoic acid. Alternative splicing of the C-terminal regions generates four GluR5 variants (GluR5-2a–d). Of these, GluR5-2a is 49 amino acids shorter than GluR5-2b, due to the introduction of a premature stop codon, producing a C-terminal domain of just 16 amino acids (Lerma, 2003). To test the effect of PKC activation, we exposed the cell being recorded (conventional patch) to the phorbol ester PdBu (1 μM) for 3 min, and compared the kainate-induced responses to those observed before PdBu application (Figure 4A). The amplitude of kainate-induced responses in cells transfected with GluR5-2a and KA2 subunits did not decline after the PdBu pulse (5.4±2.9% of attenuation, n=5). In contrast, GluR5-2b-containing receptors displayed a significant reduction in their response amplitude after PKC stimulation. Furthermore, this decline was larger for homomeric GluR5-2b (54.2±3.8%, n=4) than for heteromeric GluR5-2b/KA2 (28.1±3.1%, n=12) receptors (Figure 4B). As a control for phorbol ester specificity, we repeated the experiment with 4α-PdBu (1 μM, 3 min), which is inactive to activate PKC. This compound did not significantly stimulate rundown of GluR5-2b homomeric receptors. To ascertain whether repetitive activation of recombinant KARs recapitulates the properties observed in native receptors, we applied pulsed of kainate (50 μM, 1.5 s) and measured rundown of KAR-mediated currents under perforated patch-clamp configuration. As it was observed in DRG neurons, response amplitude in SHSY5-Y cells showed a time-dependent decline in amplitude (58.4±27.8% in 6 min, n=3), which was prevented by treating the cells with pertussis toxin (Figure 4B, inset). These results demonstrate that the properties of native KARs could be reproduced in a recombinant system. Moreover, they show that not all subunits are targeted by PKC such that the PKC-induced rundown is particularly marked in the 2b variant of the GluR5 subunit. Therefore, we decided to carry out further experiments on homomeric GluR5-2b receptors.
Figure 4.
PKC-mediated rundown of recombinant KAR responses to KA in the neuroblastoma cell line SHSY5-Y. (A) Under the conventional whole-cell configuration, cells transfected with different combinations of KAR subunits were exposed to the phorbol ester PdBu (1 μM) for 3 min, and the degree of current attenuation was determined. (B) The maximum level of rundown was seen with homomeric GluR5-2b receptors, on which the inactive analogue of phorbol ester, 4α-PdBu, had no action. Bars represent the degree of rundown observed for the receptors as the mean±s.e.m. of 4–12 experiments. The inset shows that under perforated whole-cell configuration, repetitive activation of GluR5 induced a G-protein-dependent rundown of kainate-induced responses, indicating the reproduction in a heterologous system of the main properties of KAR signalling observed in neurons.
PKC-mediated phosphorylation of GluR5 induces the internalization of KARs
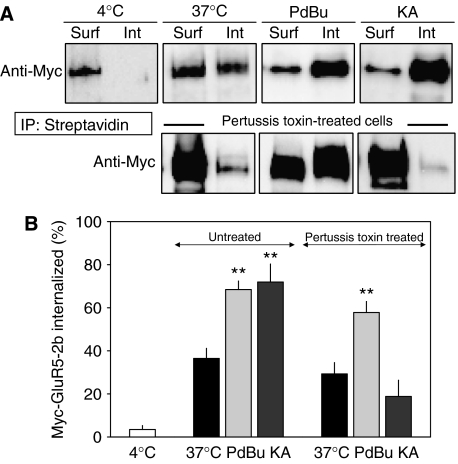
Phosphorylation of receptor channels has classically been associated with a change in the gating properties of the channel itself (for a review see Swope et al, 1999). Such an effect would justify a change in the whole-cell current levels upon PKC activation. However, more recent results have pointed out that phosphorylation of membrane receptors may be a signal for their endocytosis (Barry and Ziff, 2002). Therefore, since GluR5-2b subunits can be phosphorylated by PKC, the use-dependent rundown of KAR mediated by PKC could be due to either of these phenomena (Hirbec et al, 2003). To determine whether PKC triggers endocytosis of GluR5-2b subunits, we assayed the internalization of biotinylated surface myc-tagged recombinant GluR5-2b expressed in SHSY5-Y cells. After biotinylating all surface proteins for 15 min at 4°C, the cells were returned to growth medium at 37°C for 3 min to allow endocytosis to proceed. The cells were then lysed and the membrane and soluble fractions separated by centrifugation to separate the biotinylated proteins that remained on the surface of the membrane from the internalized proteins. Controls were performed to ensure that biotin did not access the intracellular compartment during incubation, and that the membrane fraction did not contaminate soluble proteins (Supplementary Figure 1). The amount of biotinylated GluR5-2b was determined by binding the cell lysate to streptavidin–agarose beads and probing Western blots with an anti-myc antibody. There was negligible endocytosis in cells left at 4°C (3.5±1.8%, n=9; Figure 5), whereas there was significant internalization of GluR5-containing KARs after 3 min under basal conditions at 37°C (36.5±4.6%, n=8). When cultured cells were exposed to phorbol esters during this period to stimulate PKC activity, internalization was enhanced such that 68.5±3.9% of the biotinylated KARs were recovered from intracellular compartments. Interestingly, a 3-min exposure of KARs to kainate (300 μM) dramatically accelerated the internalization process, reaching a value of 71.9±8.4% (Figure 5A and B). The treatment of transfected cells with pertussis toxin (1 μg/ml, 3 h, 37°C) before carrying out the biotinylation assays abolished the internalization induced by kainate (18.8±7.5%, n=8), but not that induced by PKC activation (57.8±5.1, n=4), indicating that kainate activity is G-protein dependent.
Figure 5.
KAR non-canonical signalling induces internalization of KARs. (A) Myc-tagged GluR5-2b expressed in SHSY5-Y neuroblastoma was biotinylated before exposure to PdBu (1 μM) or kainate (300 μM) for 3 min. As a control, basal internalization was estimated at 4 and 37°C. Soluble and membrane fractions were separated, immunoprecipitated with streptavidin, immunoblotted, and probed with the myc antiserum. Fractional internalization was calculated by taking the sum of the signals present in plasma membrane (surface, Surf) and soluble fractions (internalized, Int) as 100%. In one set of experiments, cells were exposed for 3 h to pertussis toxin (0.5 μg/ml), before running the assay. (B) Summary of experimental data (n=4–11). **P<0.01; Student's t-test.
These results demonstrate that rather than affecting the gating properties of KAR channels, the phosphorylation of GluR5-2b receptors or intermediate proteins by PKC stimulates their internalization. Moreover, KAR activation triggered this process through the activation of a G-protein, that is, through the non-canonical pathway.
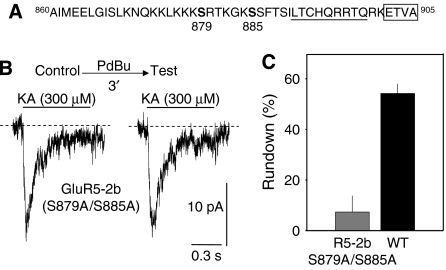
GluR5-2b contains at least six serine residues in its C-terminal domain that are consensus phosphorylation sites for PKC. However, 90% of GluR5-2b phosphorylation by PKC has been shown to accumulate at two of these residues (Hirbec et al, 2003; Figure 6A). Therefore, by mutating these two residues we assessed whether phosphorylation of GluR5-2b was responsible for the rapid internalization of KARs. Although GluR5-2b(S879A/S885A) cDNA was poorly expressed, making biochemical assays difficult, mutated receptor activity could be detected by electrophysiological recordings. In contrast to intact receptors, kainate-induced responses of these mutants were markedly desensitizing (Figure 6B) and a seven-fold acceleration of the decay was observed (117.8±15 ms versus 858±85 ms of desensitization time constant). More interestingly, the amplitude of the responses was not affected by a pulse of PdBu (7.3±6.4% rundown, n=10) as it was for WT subunits (e.g., 54%). However, since this mutant receptor rendered small inward currents, we wondered whether this could reflect a dependence of the non-canonical signalling on the ionotropic response. Then, we analyzed data in search for any correlation between the observed degree of rundown and the magnitude of the ionotropic current per cell recorded in experiments illustrated in Figure 1. No correlation was seen between the degree of ionotropic activity and the extent of response rundown (r2=0.2; not shown). This result is in keeping with the idea that ionotropic and metabotropic activities of KARs are independent processes of the same receptor complex (Rozas et al, 2003).
Figure 6.
Mutation of PKC phosphorylation sites in the C-terminal domain of GluR5-2b abolishes receptor rundown. (A) Amino-acid sequence of the C-terminal domain of GluR5-2b. Serine targets for PKC phosphorylation are shown in bold. Underlined residues correspond to the ER retention signal identified, and the boxed region is the PDZ binding domain. (B) Examples of KA-induced responses in GluR5-2b double mutants expressed in SHSY5-Y cells before and after a 3 min exposure to PdBu. (C) PdBu-induced rundown of mutated GluR5-2b was minimal (n=10 cells). For comparison, the rundown obtained from wild-type construct (as in Figure 4B) is also shown in this graph.
These results indicate that PKC-mediated phosphorylation of S879 and/or S885 of the GluR5-2b subunits is responsible for triggering the rapid internalization of KARs.
Discussion
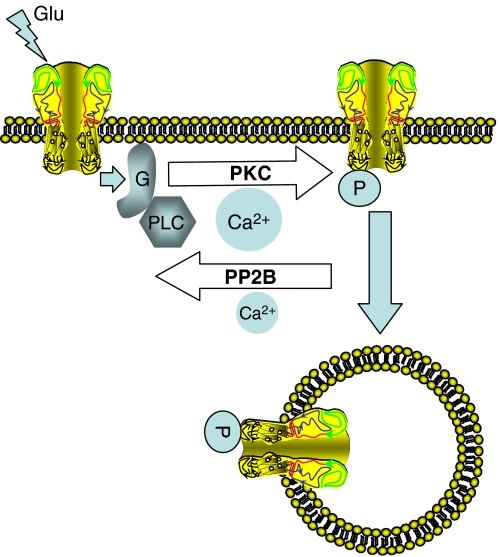
The data presented here indicate that the number of GluR5-containing KARs at the plasma membrane is dynamically regulated in an activity-dependent manner. This regulation appears to be controlled by PKC phosphorylation of GluR5, which represents a key signal for receptor internalization. In particular, two serines present in the C-terminal domain of GluR5-2b (S879 and S885; Hirbec et al, 2003) act as effectors for the PKC-triggered endocytosis of surface KARs. Furthermore, we show that the non-canonical KAR-signalling pathway may activate PKC (Rodriguez-Moreno and Lerma, 1998; Rozas et al, 2003). Indeed, KARs trigger PKC activation through a G-protein, which leads to the endocytosis of surface receptors. This kind of signalling serves as a sensor for KAR activity, probably acting as a feedback mechanism to limit neuronal overactivation through the KAR system. Thus, the dual signalling system conveys a discriminative capacity to the KARs, such that their repetitive activation may result in a rapid downregulation of the number of receptors in the membrane capable of being activated (see Figure 7 for an illustrative model).
Figure 7.
A model of KAR autoregulation. KAR activation by the ligand triggers both an ion current and the G-protein-mediated stimulation of PKC, which in turn phosphorylates the KAR C-terminal domain. Phosphorylated receptors are more likely to be endocytosed, inducing an activity-dependent rundown of ion channel-mediated responses. Ca2+–calmodulin-dependent phosphatase 2B (calcineurin) dephosphorylates KARs, reversing the action of PKC. Both activities establish an equilibrium that is modulated by internal Ca2+, such that large concentrations favor PKC activity, whereas low concentrations allow calcineurin activation without further supporting PKC.
An interesting aspect raised by the data presented is that the dual signalling capacity of KARs can be reproduced in a recombinant system by simply expressing GluR5-2b subunits. In cultured DRG cells, the activation of KARs produces the release of Ca2+ from intracellular stores in a G-protein-dependent manner, and the concomitant activation of PKC inhibited voltage-dependent N-type calcium channels (Rozas et al, 2003). This non-canonical signalling is independent of ion channel activity, but both pathways depend on a common ionotropic subunit, GluR5. However, it has recently been proposed that the KA2 subunit is critical to trigger the activation of G-proteins (Ruiz et al, 2005), possibly participating in a heteromeric complex with GluR6 subunits (Fisahn et al, 2005). Since the initial description in GABAergic terminals (Rodriguez-Moreno and Lerma, 1998), G-protein-linked KAR signalling has been described in many synapses and systems. These include sensory neurons (Rozas et al, 2003), inhibitory presynaptic terminals (Cunha et al, 2000; Delaney and Jahr, 2002; Maingret et al, 2005), excitatory presynaptic terminals (Schmitz et al, 2000; Frerking et al, 2001; Lauri et al, 2001) and in excitatory postsynaptic sites (Melyan et al, 2002; Fisahn et al, 2005; Ruiz et al, 2005). Taking into account that each of these systems expresses different sets of KAR subunits, it is conceivable that KAR subunits are promiscuous, and that several if not all of them can interact with and activate G-proteins. Unfortunately, how an ion channel-forming subunit could interact and activate a G-protein is not yet clear. Together, these results support the idea that one or several KAR-interacting proteins, rather that a KAR subunit itself, are responsible for this coupling. Our data indicate that GluR5 subunits at least are able to stimulate PKC activity through the activation of a G-protein.
Electrophysiological experiments on native receptors demonstrated that the reduction of KAR signalling upon repetitive stimulation falls to a plateau of around 40–50%. Less frequent activation of KARs seemed to allow complete recovery of the response, indicating that the number of receptors in the membrane is in dynamic equilibrium. Recovery of membrane KARs depends on calcineurin activity, since it was abolished by specific calcineurin inhibitors. Thus, we hypothesised that any imbalance between kinase and phosphatase activity would result in the rapid downregulation of the KAR-mediated response. Indeed, activity-dependent rundown of KARs seems to depend critically on two serine residues in the C-terminal domain that are targets for PKC phosphorylation (Figure 6). In addition, downregulation of KARs is also affected by changes in the levels of intracellular Ca2+. Since both PKC and calcineurin are regulated by Ca2+ (Klee et al, 1979), this is an element that may disturb the balance between the two systems. Calcineurin is a Ca2+-dependent phosphatase with an affinity for this cation one order of magnitude higher than that of PKC (half maximal activation attained at ∼0.3 μM and ∼5 μM, respectively; see Stewart et al, 1982; Perrino et al, 1995; Kohout et al, 2002). Thus, at lower Ca2+ concentrations, calcineurin activity is not only permitted but also favored over PKC activity. On the other hand, PKC could still be stimulated at Ca2+ concentrations at which calcineurin is saturated. Perhaps for this reason, rundown of KARs is favored at higher Ca2+ concentrations, whereas recovery is more important at lower Ca2+ concentrations. In addition, at Ca2+ concentrations approaching zero, a situation likely accomplished after BAPTA treatment, calcineurin is not expected to be active for long, given its strict requirement of Ca2+ (e.g., Stewart et al, 1982). For this reason, rundown of KARs was likely observed under this circumstance. Along these ideas, stimulation of NMDA receptors depresses KAR-mediated currents in cultured neurons (Ghetti and Heinemann, 2000). The internalization of GluR6 KARs is strongly Ca2+ dependent (Martin and Henley, 2004), although curiously, Ca2+ chelation has no effect on the process. This paradox establishes a fundamental difference between GluR5 and GluR6 KARs in terms of regulation of their membrane availability, indicating differences in the activity-dependent removal of membrane receptors. This scenario would be similar to the specific activity-dependent removal of particular AMPAR subunits, a factor that changes the AMPAR type and hence, synaptic properties after LTP (e.g., Barry and Ziff, 2002).
When compared with the electrophysiological data, biotinylation experiments directly estimated receptor internalization and indicated that higher levels (60–70%) of internalization were induced by either direct KAR activity or direct activation of PKC. These data indicate that there is a significant constitutive delivery of KARs, such that endocytosed receptors may return to the membrane, establishing a steady state. In addition, we noted substantial basal internalization of GluR5 receptors (ca. 36% over 3 min in the absence of any stimuli, at 37°C). This contrasts with the basal internalization of GluR6 recently estimated in hippocampal cultured neurons (ca. 20% in 30 min; Martin and Henley, 2004). Although these differences may be due to the different experimental model or design used, it could also indicate that different types of KARs are subjected to particular rates of constitutive turnover at the membrane. The continuous stimulation of KARs generates a substantial internalization of any type (i.e., GluR5 or GluR6) of KARs, probably by the activating PKC in both cases.
Receptor trafficking can generate LTP by quickly increasing the number of receptors in the membrane (Takahashi et al, 2003) and similarly, LTD can occur by rapid internalization of receptors (Lee et al, 2002). Different patterns of synaptic activity result in the activation of PKC, and since KARs display a dual signalling capability, PKC may be directly activated by KAR activation (Rozas et al, 2003). It was shown recently that activation of synaptic KARs produces LTD of KARs but not AMPA receptors (Park et al, 2006). Interestingly, repetitive patterns of synaptic activation in the thalamortical pathway induce LTP of AMPAR, but result in the LTD of the KAR-mediated component (Kidd and Isaac, 1999). PKC activation increases activity-induced internalization of GluR6 receptors (Martin and Henley, 2004), and we show that PKC activation is a key signal to trigger internalization of GluR5-2b-containing KARs. The activation of PKC through the non-canonical signalling of KARs may therefore be considered as a means to prevent neuronal overactivation through KARs. Contrary to what has been largely assumed, such a property makes the KAR system a poor candidate for triggering excitotoxicity. In other words, glutamate- or kainite-induced excitotoxicity would be more efficient through AMPAR or NMDAR than through KARs, the feedback mechanism in the latter preventing such effects. Indeed, recent experiments indicate that kainate induces excitotoxicity through activation of AMPAR rather than KARs (D Bredt, personal communication), clearly supporting our interpretation.
In summary, dual signalling through KARs enables these receptors to autoregulate their number in the membrane, limiting neuronal overactivation. Future experiments will determine whether this mechanism could be exploited to further control neuronal tissue excitability in diseases such as epilepsy, where such events are perturbed.
Materials and methods
All the procedures for handling and killing animals used in this study followed the European Commission guidelines (86/609/CEE) for handling experimental animals, and they were supervised by the veterinary officer at the Instituto de Neurociencias de Alicante.
DRG cell culture
DRG cultures were prepared as described in Rozas et al (2003).
Electrophysiological recordings from DRG cultured neurons and SHSY5-Y cells
Kainate-induced responses were recorded in DRG neurons after 1–2 days in culture. The internal pipette solution contained the following (in mM): 127 CsMeSO, 1 EGTA, 10 NaCl, 10 CsCl and 10 HEPES, and the external solution contained 140 NaCl, 2.5 KCl, 15 HEPES, 10 glucose, 1.8 CaCl2 and 1 MgCl2. To prevent wash-out of internal components from the cytosol, perforated whole-cell patch recordings were obtained by adding Amphotericin-B (200 μg/ml final concentration) to the solution in the pipette tip (Rae et al, 1991) and the cells were rapidly perfused as described previously (Regalado et al, 2001). In perforated patch recordings, EGTA was omitted from the internal solution and the osmolarity of the external and internal solutions was adjusted with sucrose to 320 and 300 mOsm, respectively. Kainate-induced responses were recorded similarly in SHSY5-Y transfected with different recombinant KAR GluR5 subunits. Cells were electroporated and then plated onto poly-lysine-treated coverslips at low density (∼30 000 per coverslip), and recorded 1–2 days after. Experiments in untransfected cells demonstrated the lack of expression of glutamate receptors in this cell line (not shown). The internal pipette solution contained 137 mM CsMeSO, 1 mM EGTA, 10 mM NaCl, 10 mM CsCl and 10 mM HePES, and the external solution contained 140 mM NaCl, 20 mM TEA, 15 mM HEPES, 5 mM glucose and 2 mM CaCl2. In this case, the osmolarity of the external and internal solutions was adjusted with sucrose to 345 and 325 mOsm, respectively. In both cases, the glass pipettes had a resistance 2–3 MΩ. For whole-cell configuration, recordings were initiated immediately after breaking in. For perforated whole-cell configuration, recordings were initiated when the series resistance was <40 MΩ, which was compensated by 50–60%. Currents were filtered at 1 kHz (2-pole Butterworth filter, −12 dB/octave) and transferred at a sampling rate of 20 kHz to a personal computer for analysis and display purposes using pCLAMP software (AXON Instruments). Experiments were carried out at room temperature (22–25°C).
Calcium imaging
DRG neurons were loaded with Fluo-4 AM (2 μM, 35 min at 37°C). Ca2+ transients were measured with a Leica laser confocal unit mounted on a Leica DM LFSA microscope. Neurons were visualized with ApoL × 40/0.8 or × 63/0.9 water-immersion objectives. Images were taken every second and averaged in pairs. Images were acquired, background subtracted and photobleaching corrected using Leica LCS software. Solutions were applied to the cells under study through a 300-μm diameter flow pipe situated approximately 500 μm away from the cells, at a rate of 2 ml/min.
cDNA constructs
cDNA encoding the GluR5-2b KAR subunit tagged with c-myc were kindly provided by F Coussen and C Mulle (University of Bordeaux), while the KA2 KAR subunit was a generous gift from P Seeburg (Max Plack Institute, Heidelberg). The S879 and S885 residues of GluR5 2b subunits were mutated to alanines by PCR using the following specific primers: 5′-GAAAAAGGCAAGAACTAAGGGCAAAGCTTCTTTC-3′ (forward) and 5′-GAAAGAAGCTTTGCCCTTAGTTCTTGCCTTTTTC-3′ (reverse). The mutations were confirmed by double-strand sequencing with cDNA-specific oligonucleotide primers.
Antibodies
The commercial antibodies used in these experiments included rabbit anti-Myc (Abcam) and monoclonal mouse anti-tubulin (Abcam) antibodies.
Transient expression in SHSY5-Y cultured cells
Cells were cultured in 5 ml growth media on 60 mm culture plates, in 5% CO2 at 37°C, following standard procedures. Lipofectamine 2000 transfections with cDNA constructs encoding the different GluR5 subunits and mutant constructs were performed on 90% confluent cultures.
For electrophysiological experiments, the cells were washed 24 h after transfection, dissociated with a polished glass pipette, transferred to poly-D-lysine (250 μg/ml) glass coverslips and cultured in DMEN supplemented with 10% fetal bovine serum and 1% penicillin until they were used on the next day. Whole-cell recordings were performed in the same conditions as for DRG neurons 1–3 days after transfection.
For biotinylation assays, cells were transferred to 60-mm plates previously treated with poly-D-lysine (1 mg/ml) 24 h after transfection until they were used the next day.
Determination of surface and internalized receptors
High-density transfected cells were incubated with 100 μg/ml of the lysosomal protease inhibitor leupeptin, 1 h before biotinylation. The cells were then washed once with PBS/Ca2+/Mg2+ (10 mM phosphate buffer, 2.7 mM KCl, 137 mM NaCl, 1 mM CaCl2 and 0.5 mM MgCl2, pH 7.4) at 37°C and then cooled gradually to 4°C. Cultures were incubated at 4°C with 2 ml of biotinylation reagent (1.5 mg/ml sulfo-NHS-SS-biotin in PBS/Ca2+/Mg2+; Pierce), with gentle agitation for 15 min, and then washed three times with cold PBS/Ca2+/Mg2+. Cells were then washed once with cold quenching buffer (192 mM glycine, 25 mM Tris in PBS/Ca2+/Mg2+) and further incubated twice for 5 min with the same buffer. Cell extracts were then collected by scraping the cells into 600 μl of cell lysis buffer (10 mM Tris–HCl, 10 mM EDTA, 1% Triton-X, 0.1% SDS and protease inhibitor cocktail tablet, pH 7.4).
After washing the cells with quenching buffer, assays were carried out by incubating the cells for 3 min at 37°C in growth media to allow internalization to occur in the presence or absence of drug treatments. Cells were then washed with 4°C PBS/Ca2+/Mg2+ to block internalization, and the cell extracts were collected as described and microfuged for 20 min at 14 000 r.p.m. The pellets constituted the plasma membrane fraction of the cells; they were resuspended in precipitation buffer (50 mM Tris pH 7.4, 2 mM EDTA, 2 mM EGTA, 2% Triton-X and 0.2% SDS plus protease inhibitors cocktail tablet), sonicated for 1 min on ice and rotated at 4°C for 2 h. The supernatants, which included all cytosolic proteins, were treated as described above. Solubilized proteins were passed several times through a gauge needle and centrifuged at 100 000 g for 20 min to remove the insoluble material. Both supernatants, cytosolic fractions and plasma membrane fractions, were then incubated with 50 μl of a 1:1 slurry of streptavidin beads (Pierce) overnight at 4°C in a rotator. The beads were then washed three times with precipitation buffer and once with cold PBS. Biotinylated receptors were resolved by SDS–PAGE and transferred to nitrocellulose membranes (Amersham), which were then probed with the polyclonal c-myc antiserum (dilution 1/500) and a monoclonal anti-tubulin antibody (1/5000), the latter acting as a standard control to ensure that there was no biotinylation of intracellular proteins. The labeling of proteins was then visualized by ECL (ECL Plus; Pierce).
Quantification of Western blots was performed on a phosphoimager (Science Lab, 2001) using Image Gauge. The proportion of the receptor internalized was determined by measuring the band densities of both the biotinylated surface receptor (Surf) and that corresponding to internalized receptors (Int). Fractional internalization was calculated as the ‘Int' referred to the sum of both signals. Background was subtracted from each band before measurement.
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank Drs C Mulle and F Coussen (University of Bourdeaux) for the kind gift of plasmids expressing myc-GluR5. We also thank Dr Joan Galcerán for providing us with the SHSY5-Y neuroblastoma cell line, Ms M Llinares for technical help and Drs AV Paternain, S Vega, C Alvarez De Frutos and S Selak for sharing their expertise in electrophysiology and molecular biology. This work was supported by grants to JL from the Spanish MEC (BFI2003-00161 and BFU2006-07138); RR is and JLR was a predoctoral fellow from the Ministry of Education.
References
- Bahn S, Volk B, Wisden W (1994) Kainate receptor gene expression in the developing rat brain. J Neurosci 14: 5525–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MF, Ziff EB (2002) Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12: 279–286 [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O'Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S (1990) Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron 5: 583–595 [DOI] [PubMed] [Google Scholar]
- Bureau I, Dieudonne S, Coussen F, Mulle C (2000) Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci USA 97: 6838–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA (1997) Kainate receptors mediate a slow prostynaptic current in hippocampal CA3 neurons. Nature 388: 182–186 [DOI] [PubMed] [Google Scholar]
- Clark VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Shoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D (1997) A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389: 599–603 [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsh JC, Bernard C, Ben-Ari Y (1998) GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1: 470–478 [DOI] [PubMed] [Google Scholar]
- Cunha RA, Malva JO, Ribeiro JA (2000) Pertussis toxin prevents presynaptic inhibition by kainate receptors of rat hippocampal [(3)H]GABA release. FEBS Lett 469: 159–162 [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Jahr CE (2002) Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron 24: 475–482 [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN (2002) The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J Neurosci 1: 7425–7433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Heinemann SF, McBain CJ (2005) The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurones. J Physiol 1: 199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA (1998) Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1: 479–486 [DOI] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA (2001) Kainate receptors depress excitatory synaptic transmission at CA3CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci 21: 2958–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti A, Heinemann SF (2000) NMDA-dependent modulation of hippocampal kainate receptors by calcineurin and Ca(2+)/calmodulin-dependent protein kinase. J Neurosci 20: 2766–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (1992) Ionic Channels of Excitable Membranes, 2nd edn. Sunderland, MA: Sinauer [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Couthino V, Meyer G, Isaac JTR (2003) Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37: 625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FL, Isaac JTR (1999) Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400: 569–573 [DOI] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH (1979) Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci USA 76: 6270–6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ (2002) C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry 41: 11411–11424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Delany CJ, Clarke VR, Bortolotto ZA, Ornstein PL, Isaac TRJ, Collingridge GL (2001) Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptor-mediated synaptic transmission at hippocampal mossy fibre synapses. Neuropharmacology 41: 907–915 [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M (2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36: 661–674 [DOI] [PubMed] [Google Scholar]
- Lerma J (2003) Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4: 481–495 [DOI] [PubMed] [Google Scholar]
- Lerma J (2006) Kainate receptor physiology. Curr Opin Pharmacol 6: 89–97 [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauri SE, Taira T, Isaac JT (2005) Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. J Physiol 567: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Henley JM (2004) Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J 23: 4749–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B (2002) Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34: 107–114 [DOI] [PubMed] [Google Scholar]
- Park Y, Jihoon J, Isaac JTR, Cho K (2006) Long-term depression of kainate receptor-mediated synaptic transmission. Neuron 49: 95–106 [DOI] [PubMed] [Google Scholar]
- Perrino BA, Ng LY, Soderling TR (1995) Calcium regulation of calcineurin phosphatase activity by its B subunit and calmodulin. Role of the autoinhibitory domain. J Biol Chem 270: 340–346 [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M (1991) Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods 37: 15–26 [DOI] [PubMed] [Google Scholar]
- Regalado MP, Villarroel A, Lerma J (2001) Inter-subunit cooperativity in the NMDA receptor. Neuron 32: 1085–1096 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J (1997) Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19: 893–901 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lerma J (1998) Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20: 1211–1218 [DOI] [PubMed] [Google Scholar]
- Rozas JL, Paternain AV, Lerma J (2003) Noncanonical signaling by ionotropic kainate receptors. Neuron 39: 543–553 [DOI] [PubMed] [Google Scholar]
- Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C (2005) Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci 25: 11710–11718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA (2000) Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron 27: 327–338 [DOI] [PubMed] [Google Scholar]
- Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P (1982) Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett 137: 80–84 [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymon LA, Huganir RL (1999) Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res 33: 49–78 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R (2003) Experience strengthening transmission by driving AMPA receptors into synapses. Science 299: 1585–1588 [DOI] [PubMed] [Google Scholar]
- Wakamori M, Strobeck M, Niidome T, Teramoto T, Imoto K, Mori Y (1998) Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J Neurophysiol 79: 622–634 [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH (1993) A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci 13: 3582–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1