Abstract
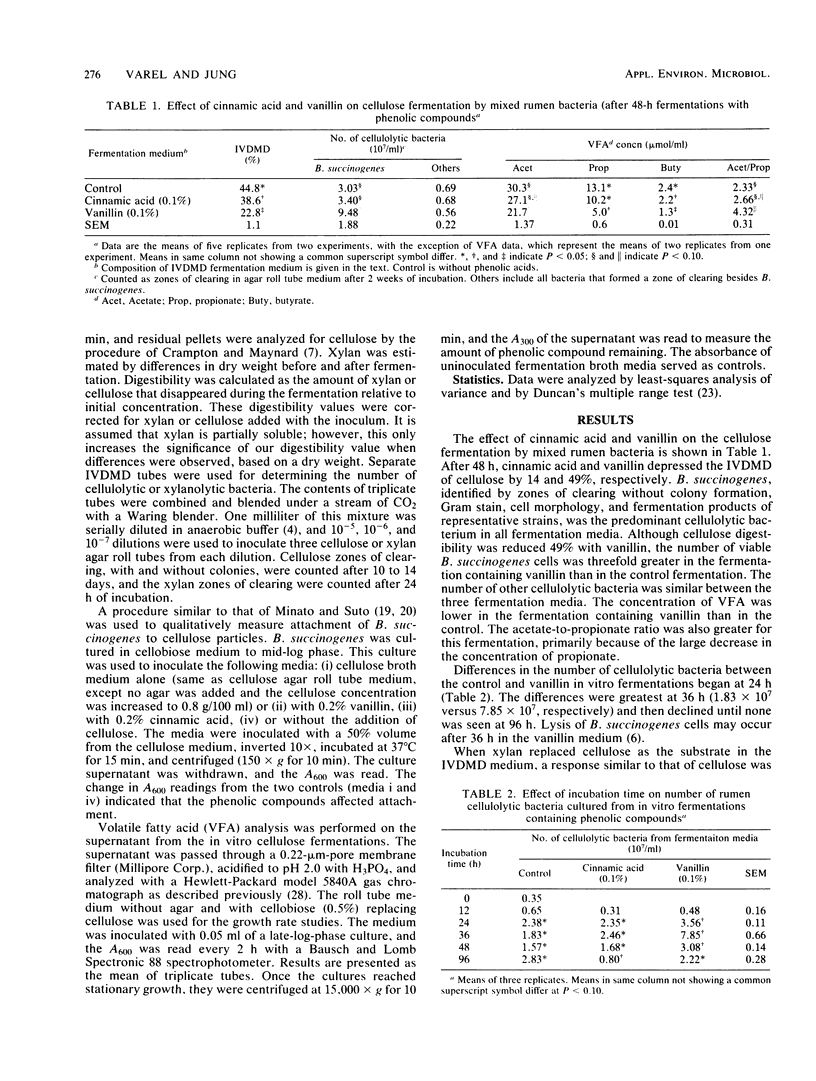
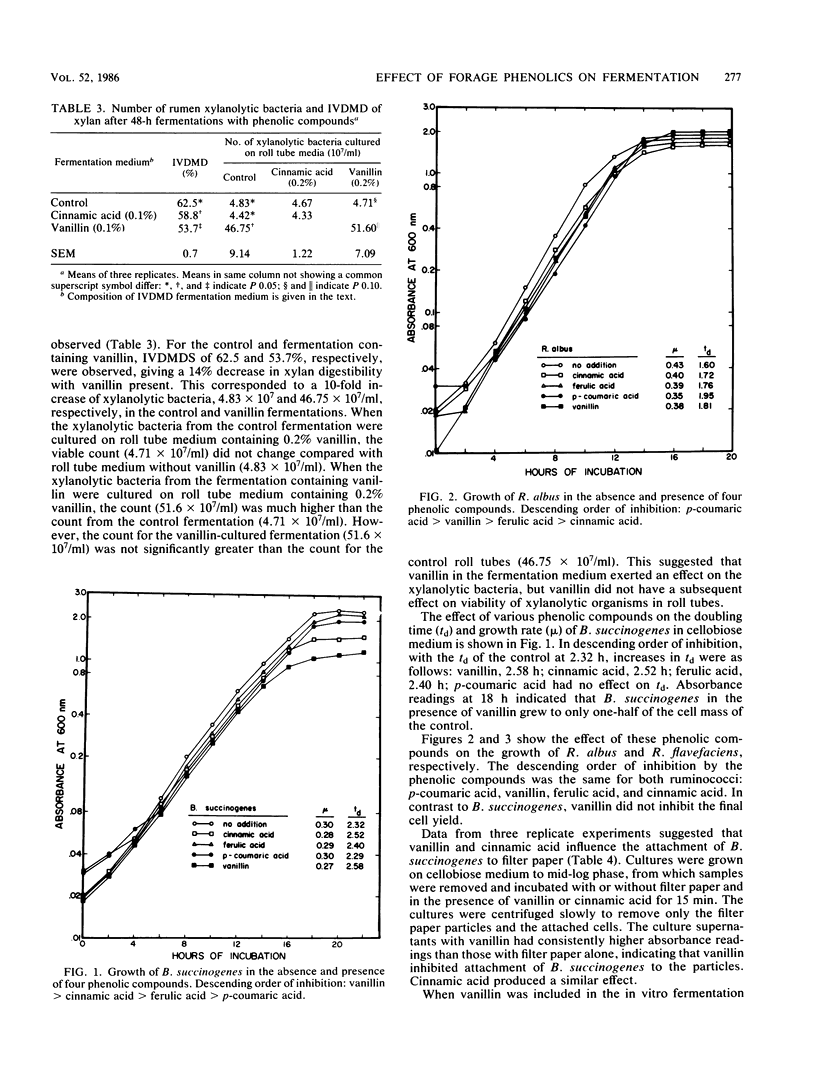
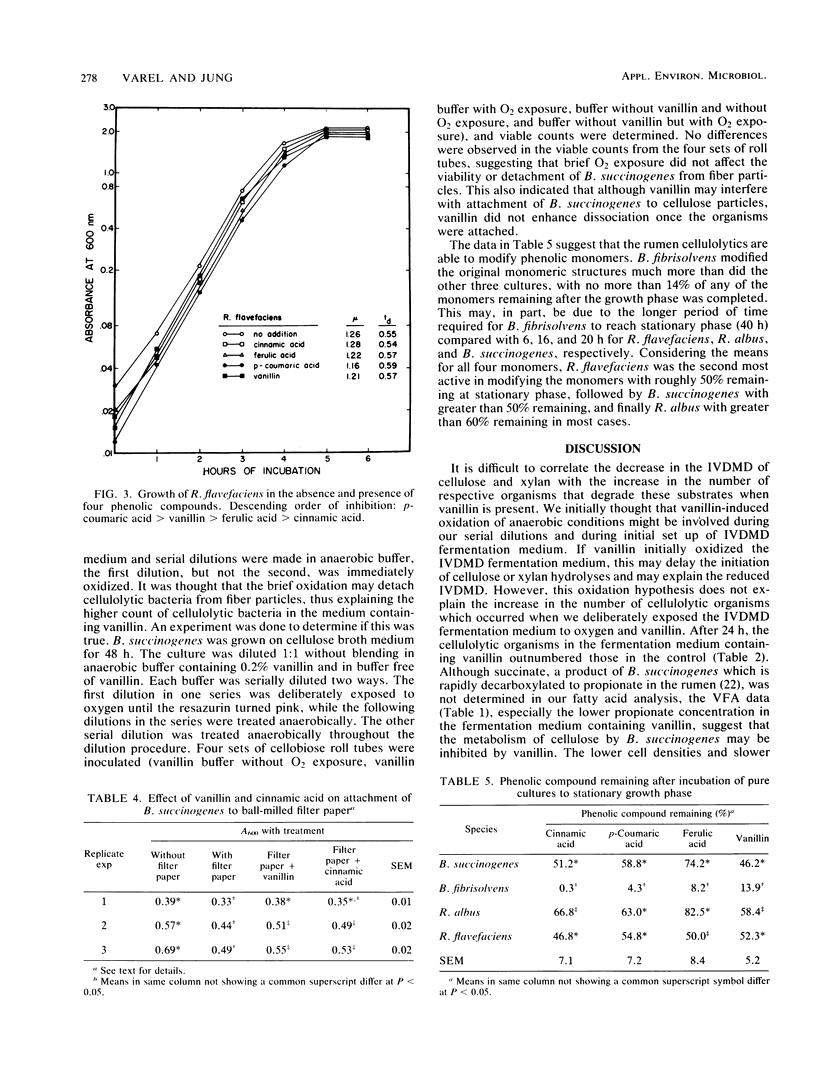
In vitro cultures of ruminal microorganisms were used to determine the effect of cinnamic acid and vanillin on the digestibility of cellulose and xylan. Cinnamic acid and vanillin depressed in vitro dry matter disappearance of cellulose 14 and 49%, respectively, when rumen fluid was the inoculum. The number of viable Bacteroides succinogenes cells, the predominant cellulolytic organism, was threefold higher for fermentations which contained vanillin than for control fermentations. When xylan replaced cellulose as the substrate, a 14% decrease in the digestibility of xylan was observed with vanillin added; however, the number of viable xylanolytic bacteria cultured from the batch fermentation was 10-fold greater than that of control fermentations. The doubling time of B. succinogenes was increased from 2.32 to 2.58 h when vanillin was added to cellobiose medium, and absorbance was one-half that of controls after 18 h. The growth rate of Ruminococcus albus and Ruminococcus flavefaciens was inhibited more by p-coumaric acid than by vanillin, although no reduction of final absorbance was observed in their growth cycles. Vanillin, and to a lesser extent cinnamic acid, appeared to prevent the attachment of B. succinogenes cells to cellulose particles, but did not affect dissociation of cells from the particles. B. succinogenes, R. albus, R. flavefaciens, and Butyrivibrio fibrisolvens all modified the parent monomers cinnamic acid, p-coumaric acid, ferulic acid, and vanillin, with B. fibrisolvens causing the most extensive modification. These results suggest that phenolic monomers can inhibit digestibility of cellulose and xylan, possibly by influencing attachment of the fibrolytic microorganisms to fiber particles. The reduced bacterial attachment to structural carbohydrates in the presence of vanillin may generate more free-floating fibrolytic organisms, thus giving a deceptively higher viable count.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Attack on lignified grass cell walls by a facultatively anaerobic bacterium. Appl Environ Microbiol. 1980 Oct;40(4):809–820. doi: 10.1128/aem.40.4.809-820.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betian H. G., Linehan B. A., Bryant M. P., Holdeman L. V. Isolation of a cellulotytic Bacteroides sp. from human feces. Appl Environ Microbiol. 1977 Apr;33(4):1009–1010. doi: 10.1128/aem.33.4.1009-1010.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Chesson A., Stewart C. S., Wallace R. J. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl Environ Microbiol. 1982 Sep;44(3):597–603. doi: 10.1128/aem.44.3.597-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. Microorganisms in the rumen of cattle fed a constant ration. Can J Microbiol. 1957 Mar;3(2):289–311. doi: 10.1139/m57-034. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J. M., Farrand J. R., Montgomery L. Cellulolytic and non-cellulolytic bacteria in rat gastrointestinal tracts. Appl Environ Microbiol. 1982 Dec;44(6):1428–1434. doi: 10.1128/aem.44.6.1428-1434.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall E. I. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J. 1948;43(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Fairchilds I. G., Zgornicki Y. D. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. 1974 Apr;27(4):678–687. doi: 10.1128/am.27.4.678-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Wolin M. J. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl Microbiol. 1973 Nov;26(5):789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gylswyk N. O., Roché C. E. Characteristics of ruminococcus and cellulolytic butyrivibrio species from the rumens of sheep fed differently supplemented teff (Eragrostis tef) hay diets. J Gen Microbiol. 1970 Nov;64(1):11–17. doi: 10.1099/00221287-64-1-11. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Fryda S. J., Robinson I. M. Cellulolytic bacteria from pig large intestine. Appl Environ Microbiol. 1984 Jan;47(1):219–221. doi: 10.1128/aem.47.1.219-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Hashimoto A. G., Chen Y. R. Effect of temperature and retention time on methane production from beef cattle waste. Appl Environ Microbiol. 1980 Aug;40(2):217–222. doi: 10.1128/aem.40.2.217-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


