Abstract
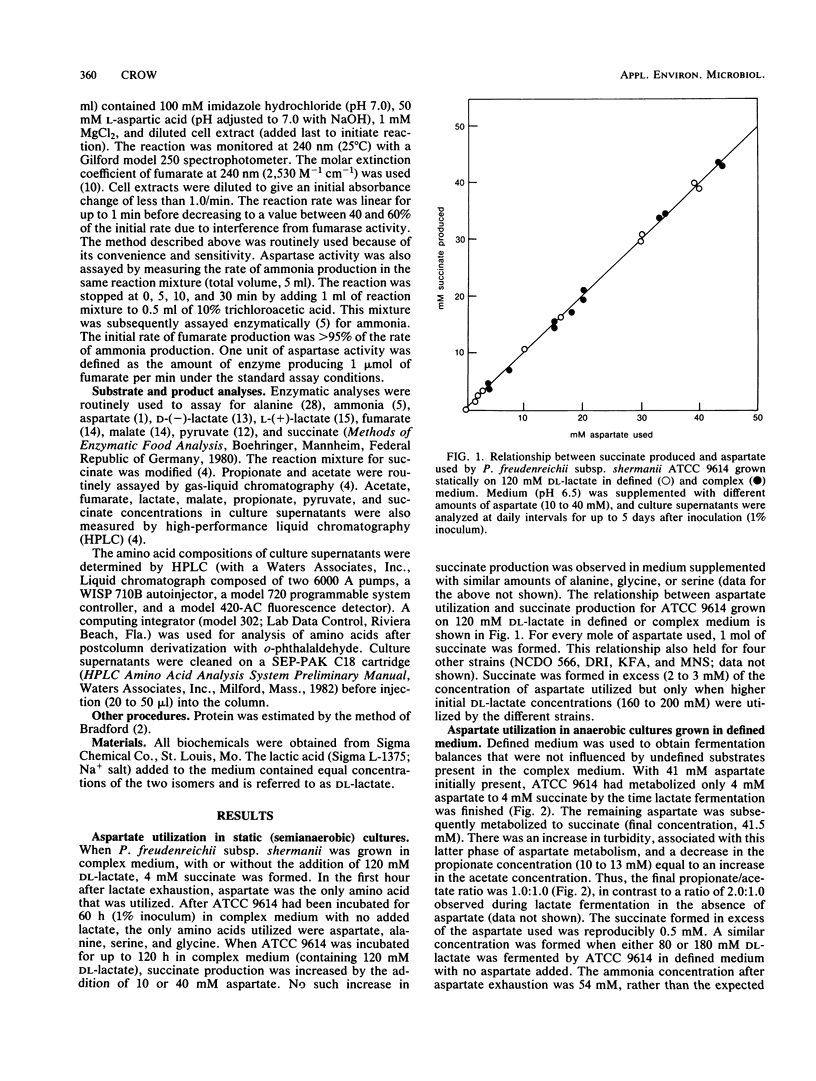
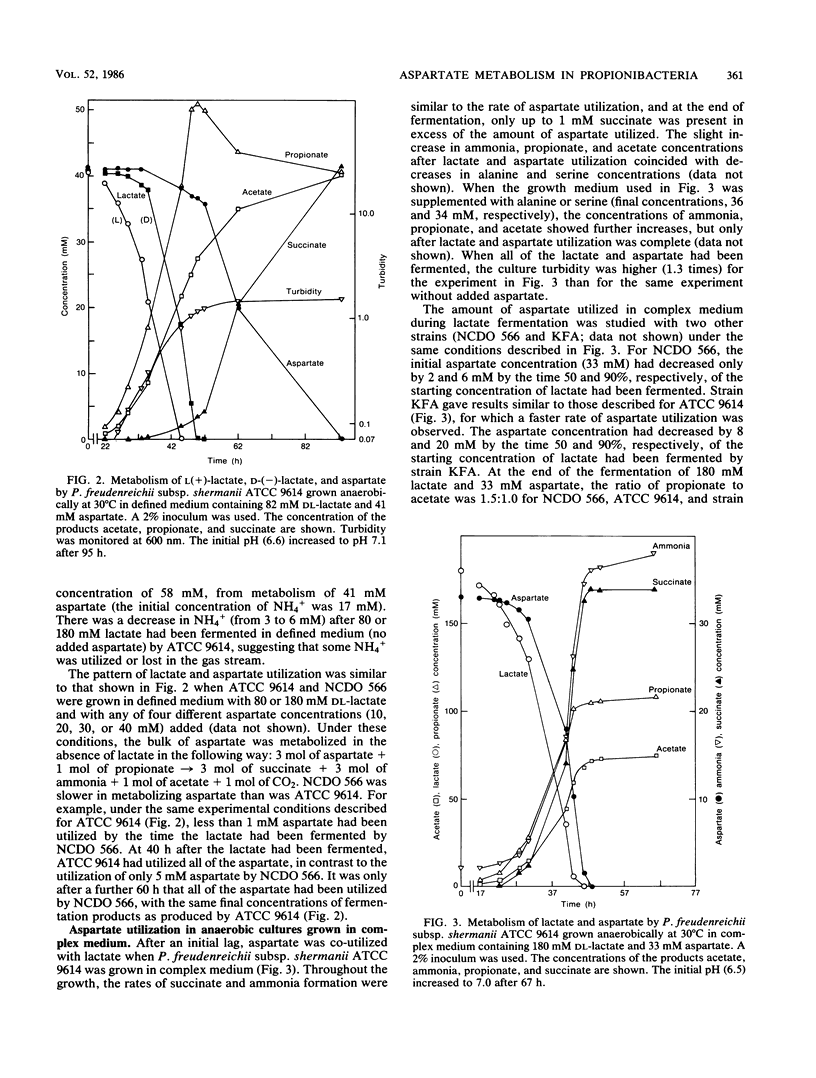
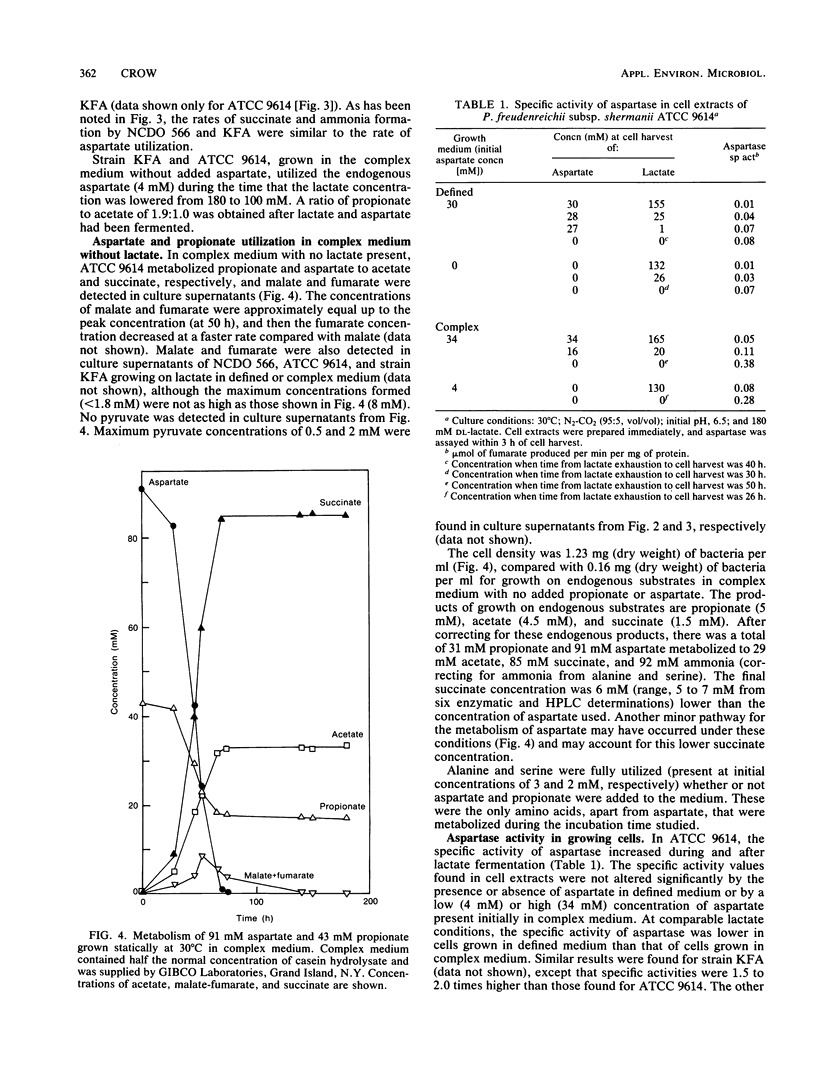
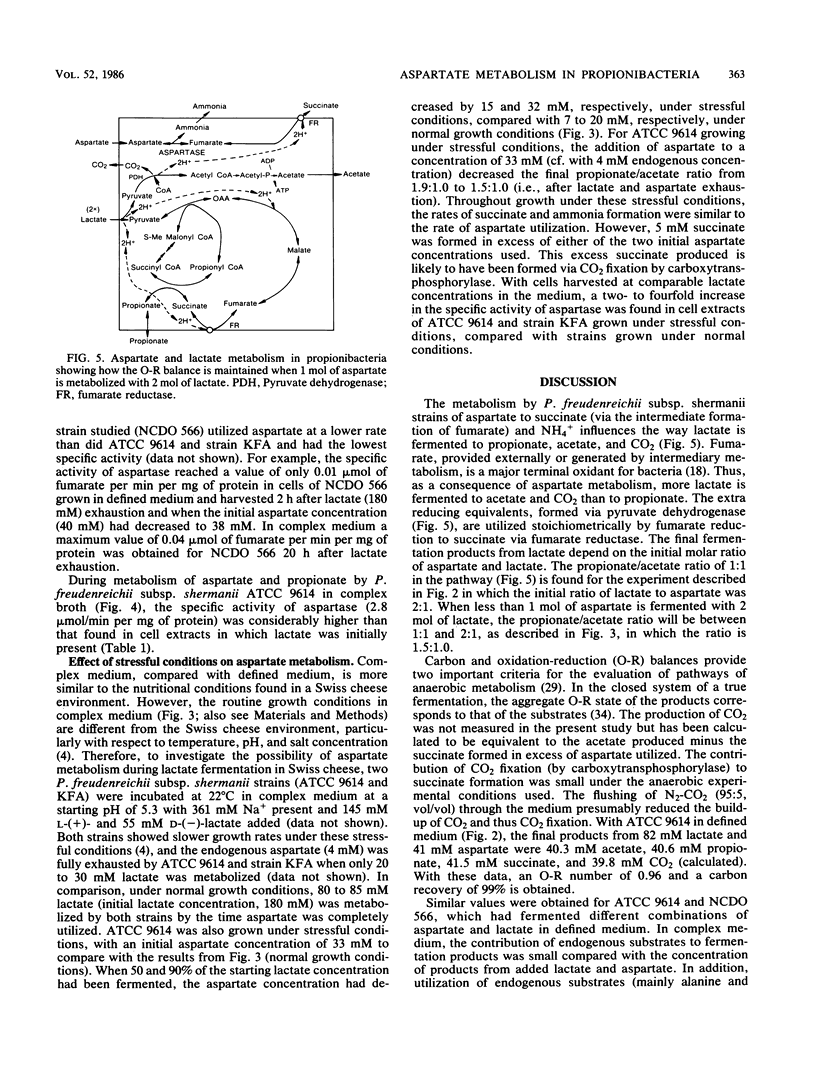
More than 90% of the aspartate in a defined medium was metabolized after lactate exhaustion such that 3 mol of aspartate and 1 mol of propionate were converted to 3 mol of succinate, 3 mol of ammonia, 1 mol of acetate, and 1 mol of CO2. This pathway was also evident when propionate and aspartate were the substrates in complex medium in the absence of lactate. In complex medium with lactate present, about 70% of the aspartate was metabolized to succinate and ammonia during lactate fermentation, and as a consequence of aspartate metabolism, more lactate was fermented to acetate and CO2 than was fermented to propionate. The conversion of aspartate to fumarate and ammonia by the enzyme aspartase and subsequent reduction of fumarate to succinate occurred in the five strains of Propionibacterium freudenreichii subsp. shermanii studied. The ability to metabolize aspartate in the presence of lactate appeared to be related to aspartase activity. The specific activity of aspartase increased during and after lactate utilization, and the levels of this enzyme were lower in cells grown in defined medium than levels in those cells grown in complex medium. Under the conditions used, no other amino acids were readily metabolized in the presence of lactate. The possibility that aspartate metabolism by propionibacteria in Swiss cheese has an influence on CO2 production is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. Arginine metabolism in lactic streptococci. J Bacteriol. 1982 Jun;150(3):1024–1032. doi: 10.1128/jb.150.3.1024-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L. Utilization of Lactate Isomers by Propionibacterium freudenreichii subsp. shermanii: Regulatory Role for Intracellular Pyruvate. Appl Environ Microbiol. 1986 Aug;52(2):352–358. doi: 10.1128/aem.52.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERY T. F. ASPARTASE-CATALYZED SYNTHESIS OF N-HYDROXYASPARTIC ACID. Biochemistry. 1963 Sep-Oct;2:1041–1045. doi: 10.1021/bi00905a022. [DOI] [PubMed] [Google Scholar]
- Fordyce A. M., Crow V. L., Thomas T. D. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl Environ Microbiol. 1984 Aug;48(2):332–337. doi: 10.1128/aem.48.2.332-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A. Fumarate as terminal acceptor of phosphorylative electron transport. Biochim Biophys Acta. 1978 Oct 23;505(2):129–145. doi: 10.1016/0304-4173(78)90010-1. [DOI] [PubMed] [Google Scholar]
- Pritchard G. G., Wimpenny J. W., Morris H. A., Lewis M. W., Hughes D. E. Effects of oxygen on Propionibacterium shermanii grown in continuous culture. J Gen Microbiol. 1977 Oct;102(2):223–233. doi: 10.1099/00221287-102-2-223. [DOI] [PubMed] [Google Scholar]
- SIU P. M., WOOD H. G. Phosphoenolpyruvic carboxytransphosphorylase, a CO2 fixation enzyme from propionic acid bacteria. J Biol Chem. 1962 Oct;237:3044–3051. [PubMed] [Google Scholar]
- Schormüller J. The chemistry and biochemistry of cheese ripening. Adv Food Res. 1968;16:231–334. doi: 10.1016/s0065-2628(08)60360-2. [DOI] [PubMed] [Google Scholar]
- WOOD H. G., STJERNHOLM R., LEAVER F. W. The metabolism of labeled glucose by the propionic acid bacteria. J Bacteriol. 1955 Nov;70(5):510–520. doi: 10.1128/jb.70.5.510-520.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G. Metabolic cycles in the fermentation by propionic acid bacteria. Curr Top Cell Regul. 1981;18:255–287. doi: 10.1016/b978-0-12-152818-8.50021-9. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. Mechanism of glucose dissimilation by the propionic acid bacteria. Biochem J. 1936 Apr;30(4):618–623. doi: 10.1042/bj0300618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. The relationship of bacterial utilization of CO(2) to succinic acid formation. Biochem J. 1940 Feb;34(2):129–138. doi: 10.1042/bj0340129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. The utilization of CO(2) by the propionic acid bacteria. Biochem J. 1938 Jul;32(7):1262–1271. doi: 10.1042/bj0321262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Wijck-Kapteijn W. M., Stouthamer A. H. Influence of oxygen on growth, cytochrome synthesis and fermentation pattern in propionic acid bacteria. J Gen Microbiol. 1972 Aug;71(3):515–524. doi: 10.1099/00221287-71-3-515. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wyck-Kapteyn W. M., Stouthamer A. H. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. J Gen Microbiol. 1973 May;76(1):31–41. doi: 10.1099/00221287-76-1-31. [DOI] [PubMed] [Google Scholar]


