Abstract
The discovery of a steadily growing number of tumor antigens (TAs) has made generic, cell-free, peptide-based cancer vaccines a possible alternative to cytokine-transfected autologous cellular cancer vaccines. The major drawback of peptide vaccines, however, is the poor immunogenicity of peptides. It is commonly thought that for the induction of an effective anticancer immune response, antigen-presenting cells (APCs) have to display TA-derived peptides to T lymphocytes. Polycationic amino acids have been employed in the past to enhance transport of proteins into cells. In a systematic study, the ability of different cationic polymers to transfer fluorescence-tagged peptides to APCs was investigated. We were able to show that several compounds enhance uptake of fluorescence-labeled peptides by APCs to different degrees. The most efficient compound identified, polyarginine (pArg), enhanced peptide delivery by more than 2 logs as compared with cells treated with peptide alone, whereas polylysine (pLys) treatment resulted in approximately 10-fold increased levels of fluorescence. Augmentation of peptide uptake was concentration-dependent, and the molecular weight of pArg or pLys also influenced peptide delivery. Furthermore, highly negatively charged peptides appear to be delivered with higher efficiency, although neutral peptides were also taken up at enhanced rates. Whereas peptide uptake mediated by pLys appears to be due to an at least transient permeabilization of cell membranes, peptide delivery in the presence of pArg may rely on endocytic processes. TA-derived peptides applied as cancer vaccines in conjunction with polycations afforded antitumor protection in animal models.
Keywords: cancer vaccine, adjuvant/immunization/peptide vaccine
Most tumor vaccines currently being used in clinical trials are based on autologous cells transfected with cytokine expression vectors (1, 2). This type of vaccine is difficult to produce, and expensive and difficult to standardize. The discovery of an ever increasing number of tumor antigens (TAs) recognized by autologous T lymphocytes in human malignancies has opened up the possibility of vaccinating patients with defined antigens against cancer (3–6). Most known immunogenic TA-derived peptides are mostly but not exclusively presented in a major histocompatibility complex (MHC) class I context, where 8- to 10-aa-long peptides bind to a cleft in the MHC molecule (7). Using a novel procedure termed “transloading” to transport small peptides into cells, our group has been able to generate powerful antitumor immune responses in mouse models using tumor cells transloaded with foreign MHC class I-matched peptides (8).
However, what the field of immunotherapy of cancer is calling for are efficacious, generic, cell-free vaccines. A big step toward this goal has recently been made by Boon and coworkers (9), who induced, although at low frequency, regression in patients afflicted with malignant melanoma by simple injection of peptides derived from the TA MAGE-3. This finding is even more remarkable because end-stage patients with advanced disease were treated. One of the major drawbacks of peptide-based vaccines, however, is the low immunogenicity of peptides per se, which may at least in part explain the low frequency of response in the study described above. Numerous adjuvant formulations have been tested to increase immunogenicity of peptides, including coadministration of granulocyte–macrophage colony-stimulating factor (GM-CSF) and peptide. These tests led to antitumor immune reactions in mice and compared favorably to a combination of peptide and standard Freund’s adjuvant (10).
Early reports describe polycationic compounds as enhancers of transport of proteins into cells (11–13). We made use of these observations in an attempt to identify novel adjuvants capable of delivering peptides efficiently to antigen-presenting cells (APCs) because APCs initiate cytotoxic T cell responses (14–16). In a systematic study, the ability of different cationic polymers to transfer peptides to cells of the APC line P388D1 as well as to primary mouse macrophages was investigated using fluorescence microscopy and a flow cytometric assay. We have identified several compounds that enhance delivery of fluorescently labeled peptides to these APCs to differing degrees. Concurrently with these investigations, the two most potent compounds, polyarginine (pArg) and polylysine (pLys), were tested for their ability to induce antitumor immune responses after vaccination with TA-derived peptides and polycations. The results of this test are described in this issue of the Proceedings (17).
MATERIALS AND METHODS
Cells.
The murine monocyte-macrophage line P388D1 was purchased from the American Type Culture Collection (TIB-63) and cultured in high-glucose DMEM (Life Technologies, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS)/2 mM l-glutamine/20 μg/ml gentamycin (Life Technologies). Bone marrow-derived APCs were obtained by flushing out femurs of DBA/2 mice. Bone marrow cells were cultured in high-glucose DMEM containing 10% FCS, 5% horse serum, 2 mM l-glutamine, and 20 μg/ml gentamycin in the presence of 200 units/ml mouse GM-CSF (Genzyme; ref. 18). Approximately two-thirds of the medium was exchanged every 24 hr for the first 5 days to remove nonadherent granulocytes and B cells (19). Both adherent and loosely adherent cells were harvested between days 8 and 10 by incubation with PBS/5 mM EDTA and seeded into 8-well microscope slides (Nunc) at a cell density of 3 × 104 cells per well. Cells were >90% positive for the antibody Mac1 (Endogen, Cambridge, MA).
Synthesis, Purification, and Modification of Peptides.
Peptide synthesis was carried out on a 0.25-mmol scale (Applied Biosystems, model 433A) using Nα-9-fluorenylmethoxycarbonyl amino acids and a preloaded trityl resin (PepChem, Tübingen, Germany). Couplings were performed in dimethylformamide using N-hydroxybenzotriazole and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (20). The following side chain-protecting groups were used: tert-butyl for Asp, Glu, and Tyr; trityl for Asn, Gln, Ser, and Thr; 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl for Arg; and butyloxycarbonyl for Lys. Assembled peptides were deprotected and cleaved from the polymer support by treatment with 92% trifuoracetic acid/4% triethylsilane/4% H2O for several hours. Peptides were precipitated by the addition of tert-butylethylether/pentane (8:2) and were purified by reversed-phase HPLC on a Vydac C18 preparative column (Vydac, Hesperia, CA). All peptides were analyzed by matrix-associated laser desorption ionization time-of-flight mass spectrometry (Lasermat, Finnigan-MAT, San Jose, CA). Molecular masses of all peptides corresponded to the calculated values.
Purified peptides were dissolved in 20 mM Hepes, pH 7.3 (pH 6.0 for peptides containing Lys residues). Two equivalents of 5-carboxyfluorescein succinimidylester (Molecular Probes) in dimethyl sulfoxide were added and incubated at room temperature for several hours. Fluorescein-modified peptides were isolated by gel filtration and subsequent reversed-phase HPLC on a Vydac C18 column and were adjusted to identical fluorescein concentrations. Again, peptide mass was confirmed by matrix-associated laser desorption ionization time-of-flight mass spectrometry. Fluorescein modification of peptides introduces a total of two negative charges at physiological pH because the fluorescein molecule itself contains a carboxyl group and the N-terminal amino group of the peptide is converted into an amide bond that is not protonated at physiological pH. Peptide sequences are displayed in Table 1.
Table 1.
Ionic charge of fluorescently labeled peptides
Assays for Peptide Uptake.
For microscopic detection of peptide uptake, bone marrow-derived APCs were seeded onto microscope slides and incubated with 40 μg of the fluorescein-labeled peptide LFEAIEGFI alone or with a mixture of peptide and 50 μg/ml pLys (average chain length = 200; pLys200; Sigma) for 30 min at 37°C. After extensive washing, cells were fixed with 4% paraformaldehyde, mounted with anti-fadent (Dako), and viewed by fluorescence microscopy (Zeiss). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (Sigma).
Flow cytometric quantitation of peptide delivery (also termed “transloading assay” in the following text) was carried out by incubating 1 × 106 P388D1 cells with peptide alone or with a combination of peptide and polycations or a mixture of peptide and histones at concentrations indicated in figure legends in a final volume of 1 ml. Peptide concentrations were kept at approximately 5 μg/ml with a final fluoresein concentration of 5 nmol/ml. Peptides were first mixed with polycations or histones in 300 μl of 10 mM Hepes, pH 7.4/150 mM NaCl. After an incubation of 30 min at room temperature, 200 μl of high-glucose DMEM containing 10% FCS was added, and the mixture was combined with 500 μl of cell suspension in high-glucose DMEM/10% FCS. In pilot experiments, an incubation time of 30 min resulted in maximum peptide uptake. Longer treatment (4 or 8 hr) did not result in a significant increase in fluorescence signal.
Before analysis, cells were washed five times with a large volume of ice-cold PBS/0.2% BSA. Cells were resuspended in 1 ml of ice-cold PBS/0.2% BSA and examined by flow cytometry (FACScan; Becton Dickinson). In some experiments, an aliquot of cells was withheld and incubated for a further 30 min at 4°C in the presence of 50 μM monensin (Calbiochem). Monensin neutralizes acidic compartments. Because fluorescence of fluorescein is highly dependent on pH, neutralization will result in increased fluorescence if a compound is located in endosomes (23). This principle was used to determine whether peptides are taken up by endocytosis under the influence of cationic polyamino acids.
The cationic polyamino acids pArg, pLys, and polyornithine at different chain lengths; Arg-rich and Lys-rich histone preparations; and protamine were purchased from Sigma. pArg polymers of 30, 25, 20, 15, and 10 residues were synthesized and purified as described above.
Lactate Dehydrogenase (LDH) Release.
Release of the cytoplasmic enzyme LDH into the cell culture supernatant was determined after incubation of P388D1 cells with peptide alone, a combination of peptide and cationic compounds, or cationic compounds alone as described above (transloading assay). Enzyme activity was measured with a commercially available kit, according the manufacturer’s instructions (CytoTox 96; Promega). All assays were carried out in triplicate; average values are shown.
RESULTS
Cationic Polyamino Acids Deliver Peptides Efficiently to APCs.
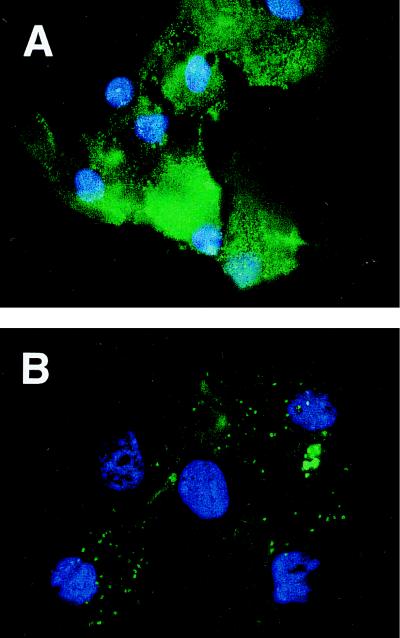
We have previously shown that pLys very efficiently enhances uptake of MHC class I-matched peptides into tumor cells and have used these “transloaded” cell preparations to generate potent antitumor immune responses (8). We and others (15, 16) have shown that APCs play a crucial role in triggering a cytotoxic anticancer T cell response by presenting TAs to T lymphocytes in lymphoid organs. Therefore, we first wanted to determine whether short peptides could be transloaded on a prototype APC. Bone marrow-derived APCs generated with GM-CSF were incubated with a combination of a fluorescence-labeled peptide plus pLys or peptide alone and viewed by fluorescence microscopy. As displayed in Fig. 1, a marked increase in uptake of fluorescence-tagged peptide by bone marrow-derived APCs is observed as compared with cells incubated with peptide alone. Whereas fluorescence appears to be sparse and particulate in cells treated with peptide alone (pulsed), intense fluorescence of transloaded cells was found not to be localized and generally to be more evenly distributed throughout the cell.
Figure 1.
Peptide delivery to bone marrow-derived APCs. Fluorescence microphotograph of bone marrow-derived APCs incubated with a mixture of fluorescein-labeled peptide LFEAIEGFI and pLys200 (A) or with fluorescein-tagged peptide alone (B). After extensive washing, cells were fixed and nuclei counterstained with 4,6-diamidino-2-phenylindole. No fluorescence was detectable in untreated control samples (data not shown).
Because the APCs were an excellent target for transloading of small peptides, we based our strategy for identifying novel adjuvants for peptide therapy on an in vitro FACS assay, allowing rapid quantification of uptake of a fluorescently labeled MHC class I binding model peptide (LFEAIEGFI, a derivative of the influenza hemagglutinin N terminus; ref. 8). An antigen-presenting mouse cell line, P388D1, was used in these experiments.
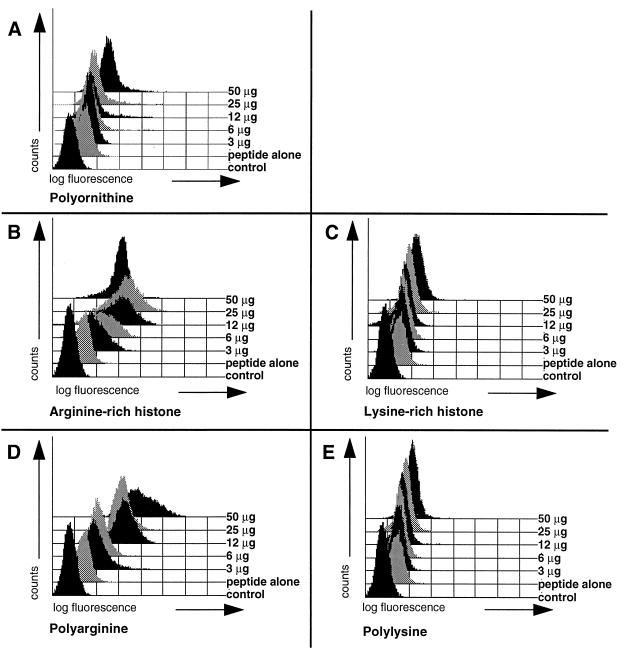
It is known that cationic polyamino acids and histones enhance uptake of proteins or even lager particles, including bacteria, into cells (11–13, 24). We systematically studied several compounds of this class for their ability to enhance peptide delivery to cells. P388D1 APCs were incubated with fluorescence-tagged peptide alone or cells were incubated with a mixture of peptide and Arg-rich histone or pArg at increasing concentrations from 3 to 50 μg/ml. In the presence of these compounds, there was a marked uptake of peptides in a concentration-dependent manner (Fig. 2 B and D). A large shift of the fluorescence signal, in the best cases by nearly 3 logs, ensued (Fig. 2A). Treatment of cells with pLys, Lys-rich histone preparations, and polyornithine together with labeled peptide results in a less pronounced shift in fluorescence. Nevertheless, fluorescence signals clearly exceed those seen for control cells pulsed with peptide alone (Fig. 2 A, C, and E). No enhancement of peptide delivery was observed after incubation of cells with protamine, a molecule reported to augment uptake of proteins into leukocytes (data not shown; ref. 25).
Figure 2.
Cationic polyamino acids and histone preparations greatly enhance peptide delivery to cells. The mouse APC cell line P388D1 was transloaded with a constant amount (5 μg/ml) of the fluorescein-tagged model peptide LFEAIEGFI alone or a combination of peptide and serial dilutions of cationic polyamino acids or a mixture of peptide and histone preparations at the concentrations indicated. Before analysis by flow cytometry, cells were washed extensively to remove free peptide. The following compounds were tested. (A) Polyornithine (average Mr = 110,000; average chain length = 580). (B) Arg-rich histone. (C) Lys-rich histone. (D) pArg490 (average Mr = 100,000). (E) pLys450 (average Mr = 94,000).
Peptide Delivery Depends on Concentration and Degree of Polymerization of Cationic Amino Acids and Is Influenced by the Charge of Peptides.
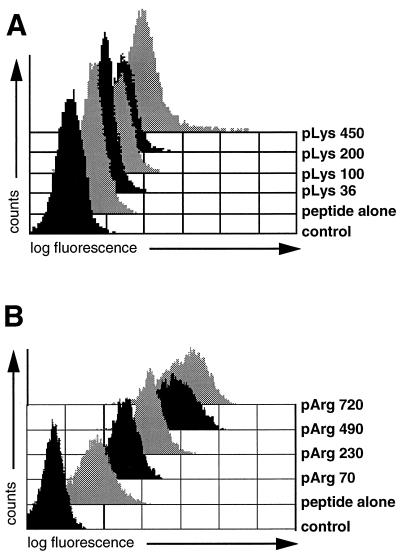
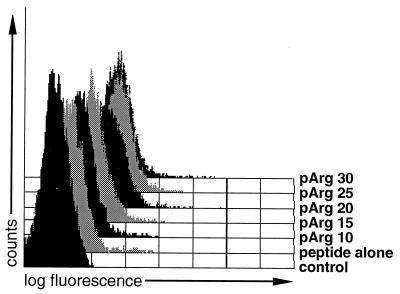
pArg and pLys were identified as the two most effective compounds; transloading mediated by polyornithine was accompanied by excessive cytotoxicity (data not shown). FACS profiles of Arg-rich or Lys-rich histone preparations were virtually superimposable with FACS profiles obtained with pArg or pLys, respectively, indicating that enhanced uptake of peptides in the presence of histones is due to their high Arg of Lys contents. The concentration and length dependence of peptide uptake mediated by pLys and pArg was determined. Again, enhancement of peptide uptake by both of these polycations is positively correlated with concentration in all cases in the range of 3–50 μg/ml (Fig. 2 D and E) and uptake increases with chain length (Fig. 3). It was observed that pArg, with a shift of over 3 logs compared with the control, has a wider useful range of concentration than pLys, with a maximal shift of less than 2 logs, and is more efficacious than pLys in delivering peptides (Fig. 3); pArg still efficiently allows transloading at concentrations as low as 3 μg/ml, whereas pLys concentrations of >25 μg were required for a significant shift in fluorescence (Fig. 2 D and E). Furthermore, pArg is more efficient than pLys at all chain lengths tested (Figs. 3 and 4). To establish whether there is a lower chain length limit for peptide delivery, polymers of pArg ranging from 10 to 30 residues were synthesized and analyzed for their ability to augment peptide delivery at high concentrations of the polycations (Fig. 4). Enhanced peptide delivery, although at low efficiency, was observed, starting with the shortest pArg polymer tested. Strongly augmented fluorescence signals as compared with samples incubated with peptide alone were only obtained with pArg chains of 20 residues or more. Thus, in practice, pArg chains of at least 15 amino acids are required for enhancing peptide delivery to cells.
Figure 3.
Peptide transloading depends on the degree of polymerization of pLys and pArg. P388D1 cells were treated with fluorescein-labeled peptide LFEAIEGFI and 50 μg/ml pLys polymers of different chain lengths (A) or 12 μg/ml pArg preparations of decreasing molecular weight (B), and the resulting fluorescence was analyzed by flow cytometry. Controls were untreated cells and APCs incubated with fluorescein-tagged peptide alone.
Figure 4.
Transloading with pArgs of low molecular weight. To determine the minimum chain length of pArg required for transloading, different polymers of pArg were synthesized and used at a concentration of 100 μg/ml for transloading with the fluorescein-labeled peptide LFEAIEGFI.
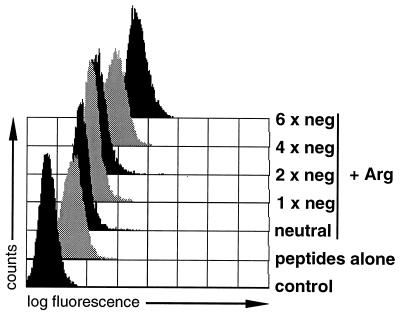
Basic polyamino acids are positively charged molecules, a fact implying that negatively charged peptides may bind to these polycations via electrostatic interactions, possibly resulting in increased peptide delivery. To test this hypothesis, the ability of cationic polyamino acids to deliver short peptides to cells as a function of charge (Table 1) was compared. Due to the procedure used to label peptides with fluorescein, tagging of peptides introduces two negative charges (see Materials and Methods). After incubation with pArg or pLys (data not shown), peptides carrying the highest number of negative charges were delivered with highest efficiency to P388D1 cells, indicating that indeed ionic interaction between peptide and cation may enhance peptide transport to cells even further (Fig. 5). Nevertheless, increased amounts of a neutral peptide were also taken up in the presence of polycations as compared with cells treated with peptide alone.
Figure 5.
Charge of peptides influences transloading efficiency. Fluorescein-labeled peptides negatively charged as indicated were compared in a transloading assay using P388D1 cells and 25 μg/ml pArg490 (average Mr = 100,000). Fluorescein contents of peptides were normalized to 5 nmol per sample. Incubation of cells with differently charged, fluorescence-tagged peptides alone resulted in similar shifts in fluorescence; only the fluorescence signal of cells incubated with the peptide LFEAIEGFI is shown. Sequences and charges of peptides are given in Table 1.
Possible Mechanisms of Peptide Uptake by APCs.
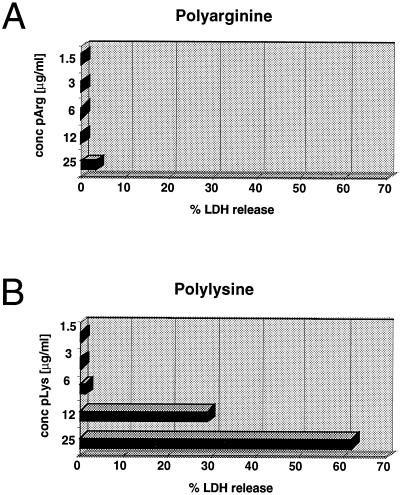
As far as the mechanism of peptide uptake mediated by basic polyamino acids is concerned, there are two extreme possibilities: (i) the compounds, perhaps transiently, permeabilize cell membranes and allow influx of the peptides, or (ii) peptides and polyamino acids are taken up by cells by endocytosis. To test the first possibility, release of the cytoplasmic enzyme LDH into the medium after incubation of cells with a mixture of pLys or pArg and peptide was measured. Whereas levels of released LDH were virtually undetectable in pArg-treated samples, high levels of LDH were measured in cell supernatants after incubation with pLys (Fig. 6). No difference in LDH release were observed in samples treated with polyamino acids alone as compared with a mixture of pLys or pArg in combination with peptide (results not shown). No measurable LDH activity was found after incubation with peptide alone (data not shown).
Figure 6.
Determination of LDH release after treatment of cells with pLys or pArg. P388D1 cells were incubated with pLys450 (average Mr = 94,000) or pArg490 (average Mr = 100,000) at the concentrations indicated using conditions identical to those for flow cytometric transloading assays, and levels of LDH released into the medium determined. LDH was not detectable in cells treated with peptide alone. No discernible difference in LDH levels was measured when cells were incubated with a combination of peptide and cationic polyamino acids (data not shown). LDH release into the medium is expressed as percentage of LDH levels of samples that underwent three freeze–thaw cycles.
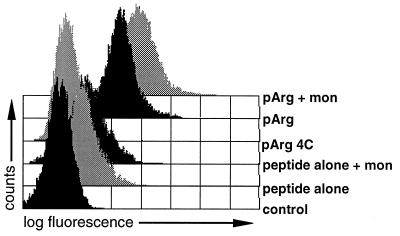
Because no LDH activity was detectable in samples treated with pArg, we tested whether in the presence of pArg peptides were taken up by endocytosis using the following procedure. A mixture of fluorescein-labeled peptide and pArg was incubated with cells for 30 min at 37°C. Samples were placed on ice and an aliquot was treated in parallel for another 30 min on ice with the drug monensin (23, 26). Monensin is known to neutralize the endosomal milieu. Because fluorescence of fluorescein is reduced at low pH, cells containing fluorescein-labeled peptides in acidic vesicles will display an increase in measurable fluorescence after incubation of the cells with monensin (23, 26). As displayed in Fig. 7, only a minor shift in fluorescence was observed in cells treated with peptide alone after monensin treatment. In contrast, fluorescence signals were greatly enhanced after monensin treatment in samples incubated with a mixture of pArg and peptide. In addition, no peptide uptake was observed when samples where incubated a 4°C, indicating that this process depends on endocytosis. As expected, only a minor shift in fluorescence was observed after monensin treatment of pLys-transloaded samples. Transloading with pLys at 4°C resulted in a measurable shift in fluorescence, again suggesting that pLys exerts its function mainly by permeabilizing cell membranes (data not shown).
Figure 7.
Peptide delivery mediated by pArg. To determine whether cells internalize peptides when transloaded with pArg, P388D1 APCs were treated with a mixture of pArg490 (average Mr = 100,000) and fluorescence-labeled peptide at 4°C or 37°C. An aliquot of sample was withheld following incubation at 37°C and treated for another 30 min on ice with 50 μM monensin before flow cytometry analysis (23, 26).
DISCUSSION
Recent investigations have indicated that the first steps in the cascade of events leading to activation and clonal expansion of T cells capable of recognizing TA peptides involve APCs, the most important of which are dendritic cells and macrophages (15, 16). Several groups have exploited these findings and made use of antigen/peptide-pulsed dendritic cells to elicit antitumor responses in mouse models (27–30) and in patients (31, 32). Most TA-derived peptides known to date are presented in a MHC class I context requiring the presence of anchor amino acids and a length of 8–10 residues (7), with CD8+ cytotoxic cells usually representing the effector cells of the antitumor immune response.
Although most TAs thus far identified are mainly derived from malignant melanoma (4), many of these appear to be expressed in other cancers as well (4–6, 33). In addition, a given tumor may express simultaneously several different TAs (4), increasing the chance of recognition of tumor cells by the immune system after vaccination with defined TAs. Since T lymphocytes recognize antigens in the form of peptides bound to MHC molecules, one form of antigen-based cancer vaccines likely will consist of a mixture of TA-derived peptides rather than a single TA-derived peptide.
In this study, we identified two compounds, pArg and pLys, that allowed very efficient delivery of peptides to APCs. The way that pLys and pArg permit entry of peptides into cells has not been entirely resolved but may differ for the two polycations. An association, possibly only fleeting, of polycation and peptide seems likely because negatively charged peptides are more efficiently transported than neutral or positively charged compounds (Fig. 5). In the case of pLys, it seems likely that permeabilization of cell membranes is involved, as seen from the leakage of LDH into the supernatant (see above). This does not appear to occur for pArg, for which the evidence points to an internalization-dependent mechanism.
In ref. 17, it is shown that a tumor vaccine consisting of a mixture of TA-derived, class I-matched peptides and pArg or pLys affords protection in animals. At present, it is unclear how the transloaded class I restricted peptides are finally bound by MHC class I molecules of APCs for presentation—a process necessary for the induction of an immune response. An increasing body of literature suggests that there are indeed pathways allowing loading of exogenous antigens/peptides onto MHC class I molecules within cells (reviewed in ref. 34).
We note that, perhaps as a consequence of the mode of entry, pLys, especially at a high molecular weight and concentration, is toxic to cells and may elicit apoptosis in an appreciable portion of cells, whereas such a behavior is not evident for pArg (results not shown). This difference, together with the more pronounced transport of peptides into APCs, may account for the generation of more potent antitumor immunity elicited by peptide-based vaccines containing pArg rather than pLys (17).
Acknowledgments
We thank Dr. Peter Steinlein for advice with flow cytometry and Drs. Margaret Chipchase and Concha Bello-Fernandez for critical reading of the manuscript.
ABBREVIATIONS
- TA
tumor antigen
- APC
antigen-presenting cell
- pArg
polyarginine
- pLys
polylysine
- pLys200
pLys with an average chain length of 200
- MHC
major histocompatibility complex
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- LDH
lactate dehydrogenase
References
- 1.Tepper R I, Mulé J J. Hum Gene Ther. 1994;5:153–164. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll D M. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 3.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon T, Cerottini J C, Van den Eynde B, van der Bruggen P, Van Pel A. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 5.Houghton A N. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins P F, Kawakami Y. Curr Opin Immunol. 1996;8:628–636. doi: 10.1016/s0952-7915(96)80078-1. [DOI] [PubMed] [Google Scholar]
- 7.Rammensee H G, Friede T, Stevanoviic S. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt W, Steinlein P, Buschle M, Schweighoffer T, Herbst E, Mechtler K, Kirlappos H, Birnstiel M L. Proc Natl Acad Sci USA. 1996;93:9759–9763. doi: 10.1073/pnas.93.18.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, et al. Int J Cancer. 1995;63:883–885. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 10.Disis M L, Bernhard H, Shiota F M, Hand S L, Gralow J R, Huseby E S, Gillis S, Cheever M A. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 11.Ryser H J, Hancock R. Science. 1965;150:501–503. doi: 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- 12.Shen W C, Ryser H J. Proc Natl Acad Sci USA. 1981;78:7589–7593. doi: 10.1073/pnas.78.12.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryser H J, Shen W C. Proc Natl Acad Sci USA. 1978;75:3867–3870. doi: 10.1073/pnas.75.8.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 15.Maass G, Schmidt W, Berger M, Schilcher F, Koszik F, Schneeberger A, Stingl G, Birnstiel M L, Schweighoffer T. Proc Natl Acad Sci USA. 1995;92:5540–5544. doi: 10.1073/pnas.92.12.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt W, Buschle M, Zauner W, Kirlappos H, Mechtler K, Trska B, Birnstiel M L. Proc Natl Acad Sci USA. 1997;94:3262–3267. doi: 10.1073/pnas.94.7.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Schwinzer R, Baccarini M, Lohmann-Mattes M-L. J Exp Med. 1989;169:973–986. doi: 10.1084/jem.169.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knorr R, Trzeciak A, Bannwarth W, Gillessen D. Tetrahedron Lett. 1989;30:1927–1930. [Google Scholar]
- 21.Sibille C, Chomez P, Wildmann C, Van Pel A, De Plaen E, Maryanski J L, de Bergeyck V, Boon T. J Exp Med. 1990;172:35–45. doi: 10.1084/jem.172.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavin M A, Gilbert M J, Riddell S R, Greenberg P D, Bevan M J. J Immunol. 1993;151:3971–3980. [PubMed] [Google Scholar]
- 23.Midoux, P., Roche, A. C. & Monsigny, M. (1993) Flow Cytometry, NATO ASI Series, ed. Jacquemin-Sablon, A. (Springer, Heidelberg), Vol. H67.
- 24.Pruzanski W, Saito S. Exp Cell Res. 1978;117:1–13. doi: 10.1016/0014-4827(78)90421-4. [DOI] [PubMed] [Google Scholar]
- 25.De Vries A, Salgo J, Matoth Y, Nevo A, Katachlski E. Arch Int Pharmacodyn. 1955;14:1–10. [PubMed] [Google Scholar]
- 26.Avrameas A, McIlroy D, Hosmalin A, Autran B, Debre P, Monsigny M, Roche A C, Midoux P. Eur J Immunol. 1996;26:394–400. doi: 10.1002/eji.1830260219. [DOI] [PubMed] [Google Scholar]
- 27.Mayordomo J I, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D, Melief C J, Ildstad S T, Kast W M, Deleo A B, Lotze M T. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 28.Paglia P, Chiodoni C, Rodolfo M, Colombo M P. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celluzzi C M, Mayordomo J I, Storkus W J, Lotze M T, Falo L D., Jr J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitvogel L, Mayordomo J I, Tjandrawan T, DeLeo A B, Clarke M R, Lotze M T, Storkus W J. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taidi B, Engleman E G, Levy R. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Chakraborty N G, Sporn J R, Kurtzman S H, Ergin M T, Mukherji B. Cancer Res. 1996;56:2479–2483. [PubMed] [Google Scholar]
- 33.Van den Eynde B, Brichard V G. Curr Opin Immunol. 1995;7:674–681. doi: 10.1016/0952-7915(95)80076-x. [DOI] [PubMed] [Google Scholar]
- 34.Jondal M, Schirmbeck R, Reimann J. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]