Abstract
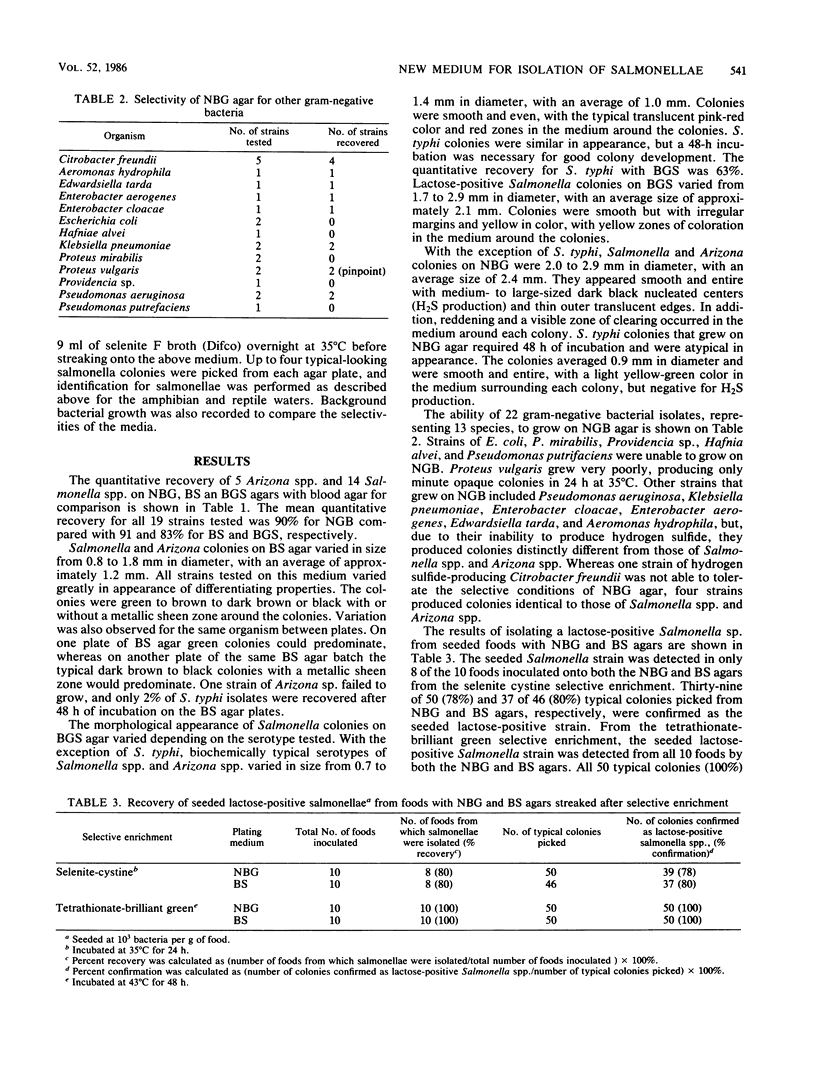
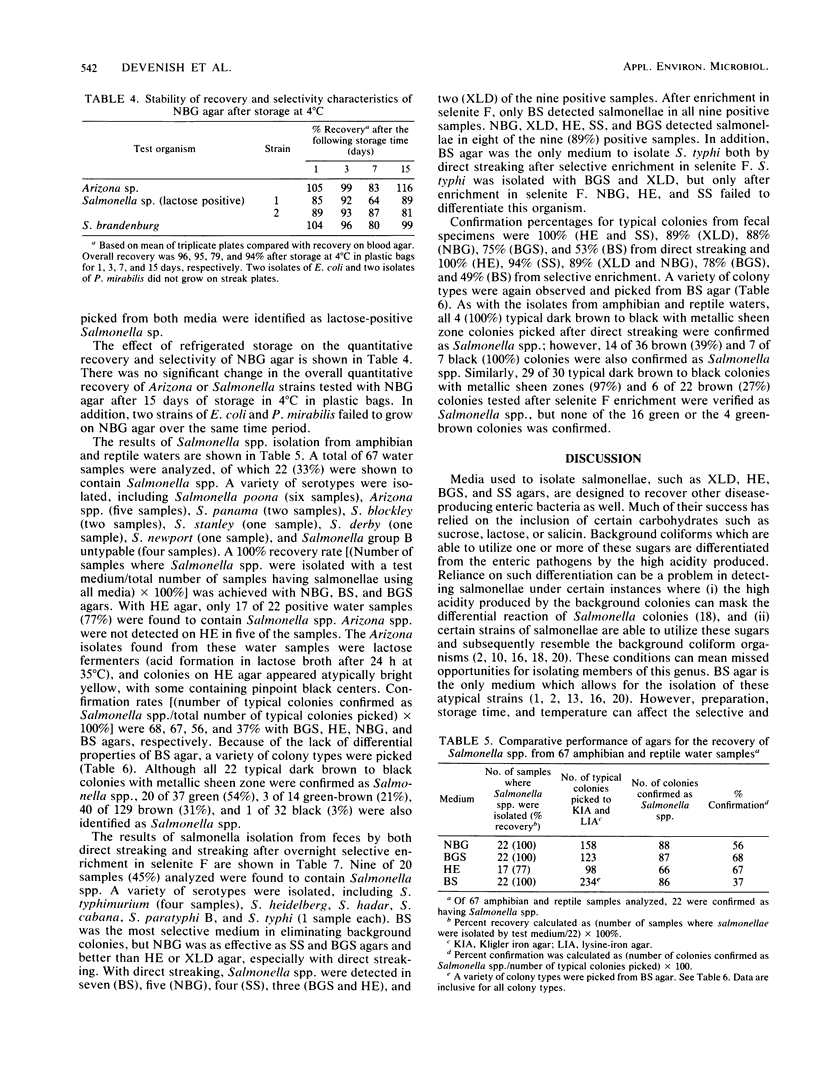
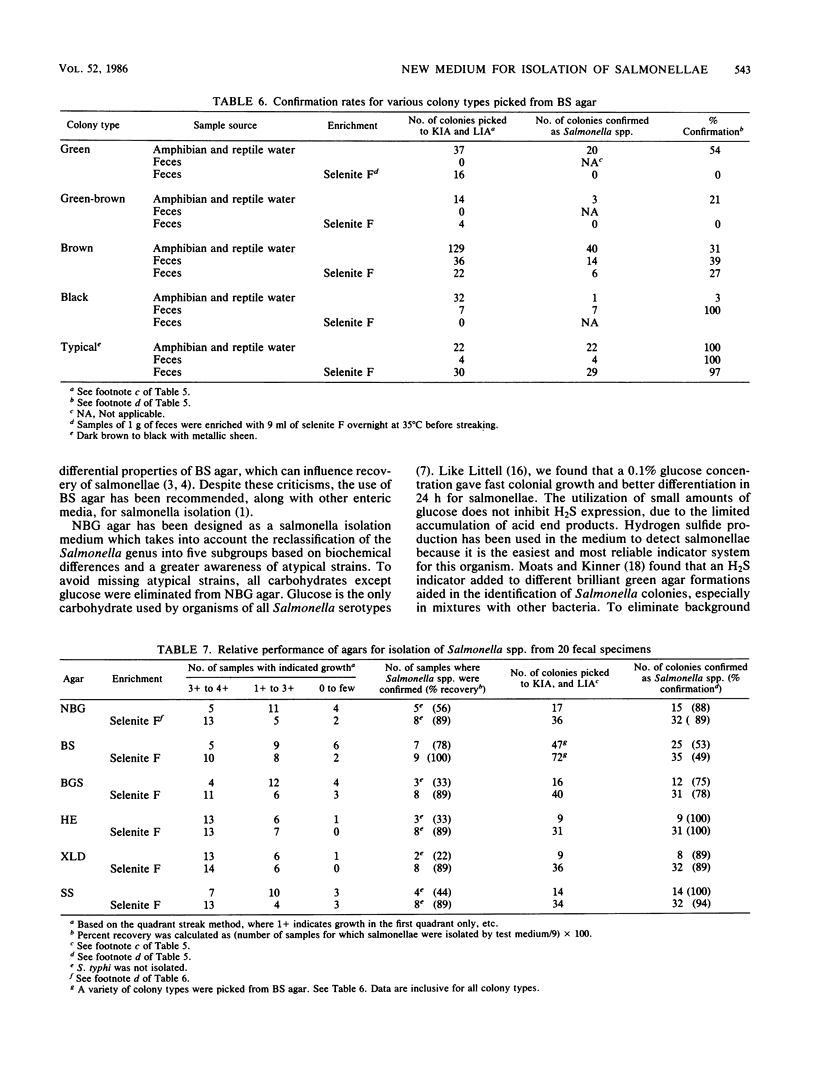
A new medium, called novobiocin-brilliant green-glucose (NBG) agar, was developed for the isolation of Salmonella spp. and evaluated against other conventionally used media including bismuth sulfite, xylose-lysine decarboxylase, brilliant green-sulfa, hektoen enteric, and salmonella-shigella agars. NBG had recovery rates comparable to the other enteric media tested with pure cultures as well as with naturally contaminated amphibian and reptile waters and fecal specimens. However, NBG, hektoen enteric, and salmonella-shigella agars failed to differentiate Salmonella typhi from a fecal specimen even after enrichment in selenite F. Although Citrobacter freundii could grow and resembled salmonellae on NBG, at no time was the recovery of Salmonella spp. colonies jeopardized by the presence of C. freundii in either seeded or naturally contaminated samples. Confirmation rates of typical colonies from NBG agar also compared favorably to the other media tested; however, bismuth sulfite, although selective, was found to have varied differential characteristics for Salmonella spp. As a result, many more colonies had to be picked, which caused bismuth sulfite agar to have the lowest confirmation rate of the media tested. The distinct advantage that NBG agar offers over the conventional method tested, including bismuth sulfite, is the consistent differential reaction of all Salmonella subgroups including biochemically atypical strains. The medium is inexpensive, easy to prepare, and can be stored for at least 2 weeks at 4 degrees C without loss of selective or differential properties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. H., Poelma P. L., Wilson C. R. Comparative efficiency of brilliant green, bismuth sulfite, Salmonella-Shigella, hektoen enteric, and xylose lysine desoxycholate agars for the recovery of Salmonella from foods: collaborative study. J Assoc Off Anal Chem. 1981 Jul;64(4):899–928. [PubMed] [Google Scholar]
- Blackburn B. O., Ellis E. M. Lactose-fermenting Salmonella from dried milk and milk-drying plants. Appl Microbiol. 1973 Nov;26(5):672–674. doi: 10.1128/am.26.5.672-674.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK G. T. Comparison of two modifications of bismuth-sulphite agar for the isolation and growth of Salmonella typhi and Salm. typhi-murium. J Pathol Bacteriol. 1952 Jul;64(3):559–566. doi: 10.1002/path.1700640317. [DOI] [PubMed] [Google Scholar]
- D'aoust J. Y. Effect of storage conditions of the performance of bismuth sulfite agar. J Clin Microbiol. 1977 Feb;5(2):122–124. doi: 10.1128/jcm.5.2.122-124.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS P. R., MCWHORTER A. C., FIFE M. A. The Arizona group of enterobacteriaceae in animals and man; occurrence and distribution. Bull World Health Organ. 1956;14(3):511–528. [PMC free article] [PubMed] [Google Scholar]
- EDWARDS P. R., MCWHORTER A. C., FIFE M. A. The occurrence of bacteria of the Arizona group in man. Can J Microbiol. 1956 May;2(3):281–287. doi: 10.1139/m56-032. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Davis B. R., Hickman-Brenner F. W., McWhorter A., Huntley-Carter G. P., Asbury M. A., Riddle C., Wathen-Grady H. G., Elias C., Fanning G. R. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985 Jan;21(1):46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A. B. Lactose-fermenting Salmonella. J Bacteriol. 1966 Apr;91(4):1661–1662. doi: 10.1128/jb.91.4.1661-1662.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoben D. A., Ashton D. H., Peterson A. C. Some observations on the incorporation of novobiocin into Hektoen enteric agar for improved Salmonella isolation. Appl Microbiol. 1973 Jul;26(1):126–127. doi: 10.1128/am.26.1.126-127.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. H., Bartlett D. I., Baker M., Dreaper R. E., Rowe B. Gastroenteritis due to Salmonella subgenus 3 (Arizona). A second case diagnosed in Britain. J Hyg (Lond) 1971 Dec;69(4):507–509. doi: 10.1017/s0022172400021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong D., Enkiri N. K., Burge W. D. Modified agar medium for detecting environmental salmonellae by the most-probable-number method. Appl Environ Microbiol. 1984 Nov;48(5):1026–1030. doi: 10.1128/aem.48.5.1026-1030.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S., Metzger W. I. A new plating medium for the isolation of enteric pathogens. I. hektoen enteric agar. Appl Microbiol. 1968 Apr;16(4):577–578. doi: 10.1128/am.16.4.577-578.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell A. M. Plating medium for differentiation Salmonella arizonae from other salmonellae. Appl Environ Microbiol. 1977 Feb;33(2):485–487. doi: 10.1128/aem.33.2.485-487.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moats W. A., Kinner J. A. Factors affecting selectivity of brilliant green-phenol red agar for salmonellae. Appl Microbiol. 1974 Jan;27(1):118–123. doi: 10.1128/am.27.1.118-123.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moats W. A., Kinner J. A., Maddox S. E., Jr Effect of heat on the antimicrobial activity of brillant green dye. Appl Microbiol. 1974 May;27(5):844–847. doi: 10.1128/am.27.5.844-847.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moats W. A., Kinner J. A. Observations on brilliant green agar with H2S indicator. Appl Environ Microbiol. 1976 Mar;31(3):380–384. doi: 10.1128/aem.31.3.380-384.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


