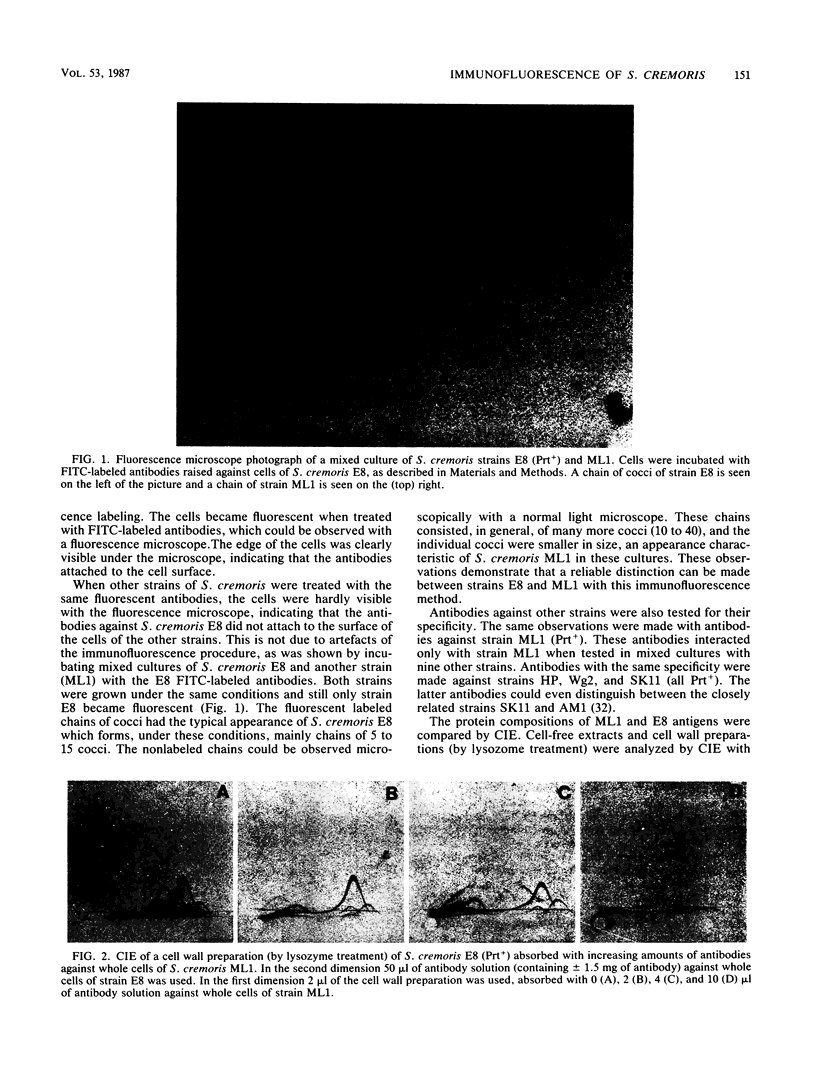
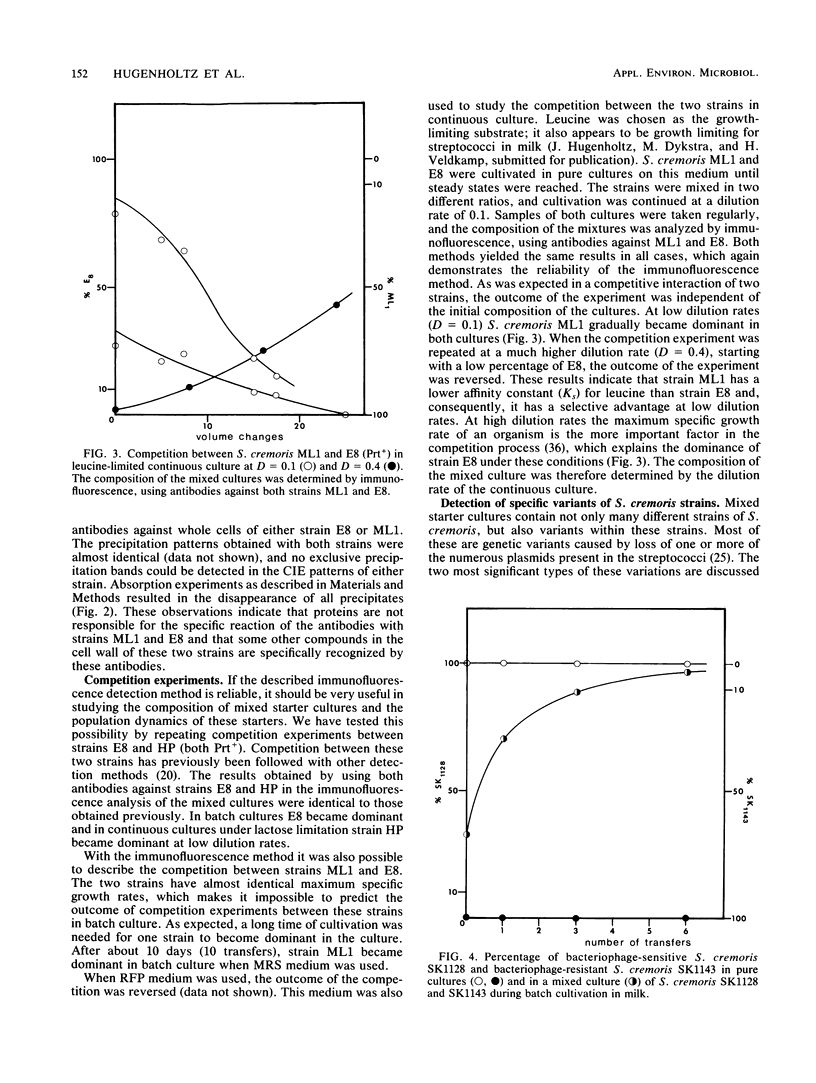
Abstract
Antisera against four different strains of Streptococcus cremoris were raised by injecting rabbits with washed suspensions of whole cells. These antisera interacted specifically with the corresponding strain in a mixture of up to nine different S. cremoris strains. The antisera could be used for analyzing the composition of mixed cultures containing these strains by immunofluorescence. Competition experiments were performed in batch and continuous cultures under amino acid limitation. A bacteriophage-sensitive variant of S. cremoris SK11 (SK1128) could be distinguished from a bacteriophage-resistant variant (SK1143) by the same immunofluorescence technique. The competition between the two variants and the stability of both variants in pure cultures were followed with the specific antibodies. Antibodies against the purified proteolytic system of S. cremoris Wg2 were used to determine the presence of proteases by immunofluorescence in several S. cremoris strains under different culture conditions. The described immunofluorescence methods can be used to analyze complex mixed starter cultures common in the dairy industry as the strains and variants present in these mixtures can be recognized microscopically.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CITTI J. E., SANDINE W. E., ELLIKER P. R. COMPARISON OF SLOW AND FAST ACID-PRODUCING STREPTOCOCCUS LACTIS. J Dairy Sci. 1965 Jan;48:14–18. doi: 10.3168/jds.s0022-0302(65)88152-8. [DOI] [PubMed] [Google Scholar]
- COLLINS E. B. Domination among strains of lactic streptococci with attention to antibiotic production. Appl Microbiol. 1961 May;9:200–205. doi: 10.1128/am.9.3.200-205.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denepitiya L., Kleinberg I. A comparison of the acid-base and aciduric properties of various serotypes of the bacterium Streptococcus mutans associated with dental plaque. Arch Oral Biol. 1984;29(5):385–393. doi: 10.1016/0003-9969(84)90165-1. [DOI] [PubMed] [Google Scholar]
- Elferink M. G., Hellingwerf K. J., Michels P. A., Seÿen H. G., Konings W. N. Immunochemical analysis of membrane vesicles and chromatophoresis of Rhodopseudomonas sphaeroides by crossed immunoelectrophoresis. FEBS Lett. 1979 Nov 15;107(2):300–307. doi: 10.1016/0014-5793(79)80395-6. [DOI] [PubMed] [Google Scholar]
- Grenier E. M., Eveland W. C., Loesche W. J. Identification of Streptococcus mutans serotypes in dental plaque by fluorescent antibody techniques. Arch Oral Biol. 1973 Jun;18(6):707–715. doi: 10.1016/0003-9969(73)90006-x. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Cell wall and serological studies on Streptococcus mutans. Caries Res. 1974;8(4):301–316. doi: 10.1159/000260120. [DOI] [PubMed] [Google Scholar]
- Hugenholtz J., Exterkate F., Konings W. N. The Proteolytic Systems of Streptococcus cremoris: an Immunological Analysis. Appl Environ Microbiol. 1984 Dec;48(6):1105–1110. doi: 10.1128/aem.48.6.1105-1110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablon J. M., Ferrer T., Zinner D. D. Identification and quantitation of Streptococcus mutans by the fluorescent antibody technique. J Dent Res. 1976 Jan;55:A76–A79. doi: 10.1177/002203457605500125011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law B. A., Kolstad J. Proteolytic systems in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):225–245. doi: 10.1007/BF00399500. [DOI] [PubMed] [Google Scholar]
- McKay L. L. Functional properties of plasmids in lactic streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):259–274. doi: 10.1007/BF00399502. [DOI] [PubMed] [Google Scholar]
- Otto R., de Vos W. M., Gavrieli J. Plasmid DNA in Streptococcus cremoris Wg2: Influence of pH on Selection in Chemostats of a Variant Lacking a Protease Plasmid. Appl Environ Microbiol. 1982 Jun;43(6):1272–1277. doi: 10.1128/aem.43.6.1272-1277.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Takahashi K. Use of fluorescamine-labeled casein as a substrate for assay of proteinases. J Biochem. 1978 Jun;83(6):1783–1787. doi: 10.1093/oxfordjournals.jbchem.a132094. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas J., Hellingwerf K. J., Seijen H. G., Guest J. R., Weiner J. H., Konings W. N. Identification and localization of enzymes of the fumarate reductase and nitrate respiration systems of escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1983 Feb;153(2):1027–1037. doi: 10.1128/jb.153.2.1027-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]