Abstract
The arginine deiminase system in a variety of streptococci and in Pseudomonas aeruginosa was found to be unusually acid tolerant in that arginolysis occurred at pH values well below the minima for growth and glycolysis. The acid tolerance of the system allowed bacteria to survive potentially lethal acidification through production of ammonia to raise the environmental pH value.
Full text
PDF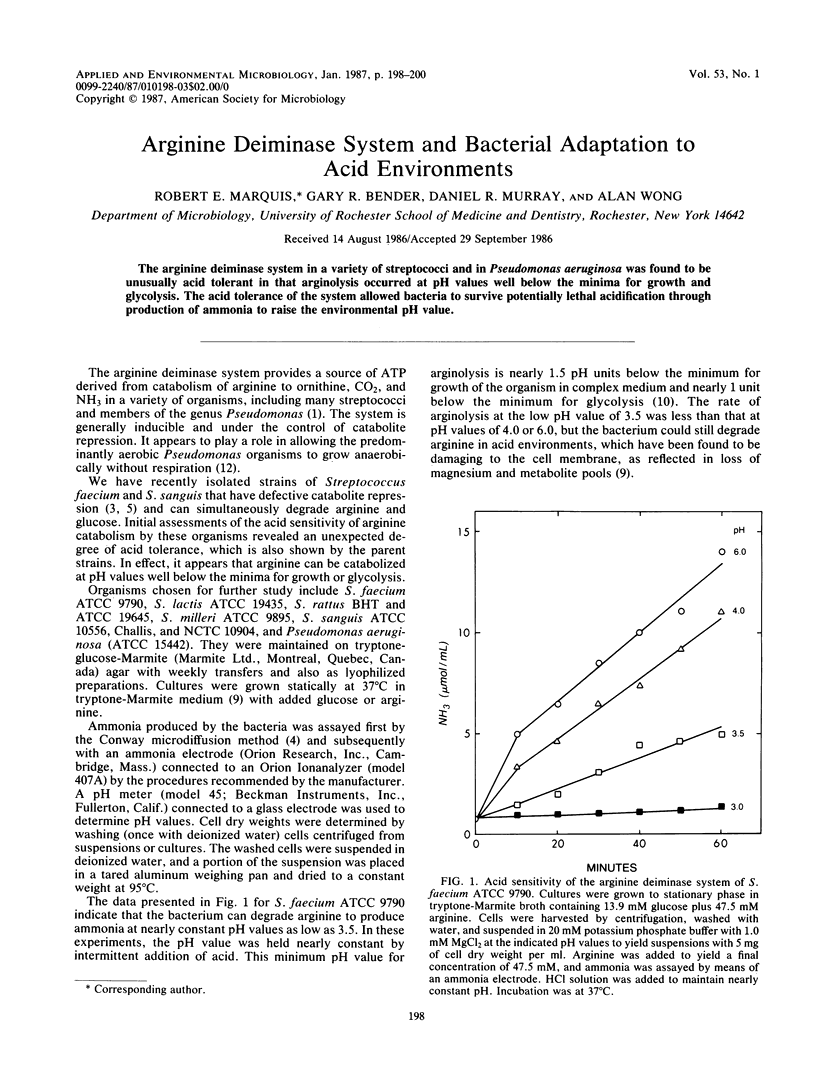


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Bender G. R., Thibodeau E. A., Marquis R. E. Reduction of acidurance of streptococcal growth and glycolysis by fluoride and gramicidin. J Dent Res. 1985 Feb;64(2):90–95. doi: 10.1177/00220345850640021701. [DOI] [PubMed] [Google Scholar]
- Campbell J., 3rd, Bender G. R., Marquis R. E. Barotolerant variant of Streptococcus faecalis with reduced sensitivity to glucose catabolite repression. Can J Microbiol. 1985 Jul;31(7):644–650. doi: 10.1139/m85-121. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapka J. A., Kleinberg I. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 1983;28(11):1007–1015. doi: 10.1016/0003-9969(83)90055-9. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Bender G. R. Isolation of a variant of Streptococcus faecalis with enhanced barotolerance. Can J Microbiol. 1980 Mar;26(3):371–376. doi: 10.1139/m80-060. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Porterfield N., Matsumura P. Acid-base titration of streptococci and the physical states of intracellular ions. J Bacteriol. 1973 May;114(2):491–498. doi: 10.1128/jb.114.2.491-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Jensen M. E. Comparison of methods for monitoring changes in the pH of human dental plaque. J Dent Res. 1982 Oct;61(10):1117–1125. doi: 10.1177/00220345820610100201. [DOI] [PubMed] [Google Scholar]
- Vander Wauven C., Piérard A., Kley-Raymann M., Haas D. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J Bacteriol. 1984 Dec;160(3):928–934. doi: 10.1128/jb.160.3.928-934.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


