Abstract
Strains of Moraxella sp., Pseudomonas sp., and Flavobacterium sp. able to grow on biphenyl were isolated from sewage. The bacteria produced 2.3 to 4.5 g of protein per mol of biphenyl carbon, and similar protein yields were obtained when the isolates were grown on succinate. Mineralization of biphenyl was exponential during the phase of exponential growth of Moraxella sp. and Pseudomonas sp. In biphenyl-supplemented media, Flavobacterium sp. had one exponential phase of growth apparently at the expense of contaminating dissolved carbon in the solution and a second exponential phase during which it mineralized the hydrocarbon. Phase-contrast microscopy did not show significant numbers of cells of these three species on the surface of the solid substrate as it underwent decomposition. Pseudomonas sp. did not form products that affected the solubility of biphenyl, although its excretions did increase the dissolution rate. It was calculated that Pseudomonas sp. consumed 29 nmol of biphenyl per ml in the 1 h after the end of the exponential phase of growth, but 32 nmol of substrate per ml went into solution in that period when the growth rate had declined. In a medium with anthracene as the sole added carbon source, Flavobacterium sp. converted 90% of the substrate to water-soluble products, and a slow mineralization was detected when the cell numbers were not increasing. Flavobacterium sp. and Beijerinckia sp. initially grew exponentially and then arithmetically in media with phenanthrene as the sole carbon source. Calculations based on the growth rates of these bacteria and the rates of dissolution of phenanthrene suggest that the dissolution rate of the hydrocarbon may limit the rate of its biodegradation.
Full text
PDF


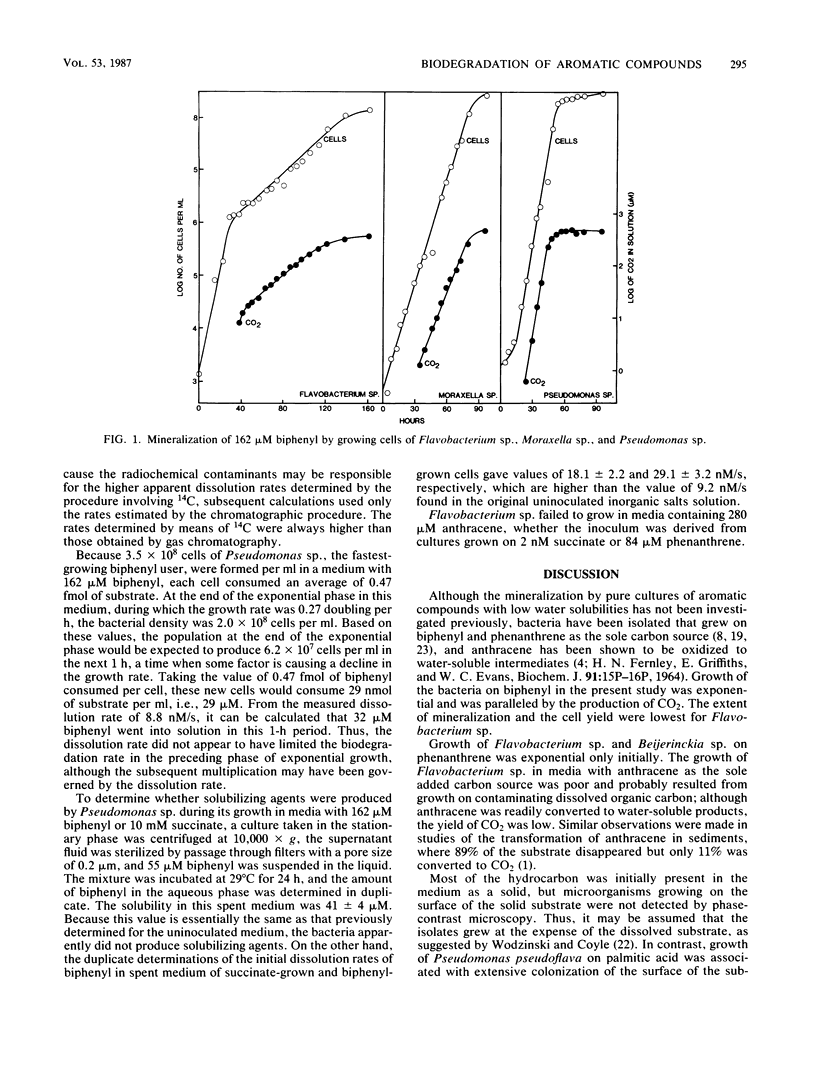
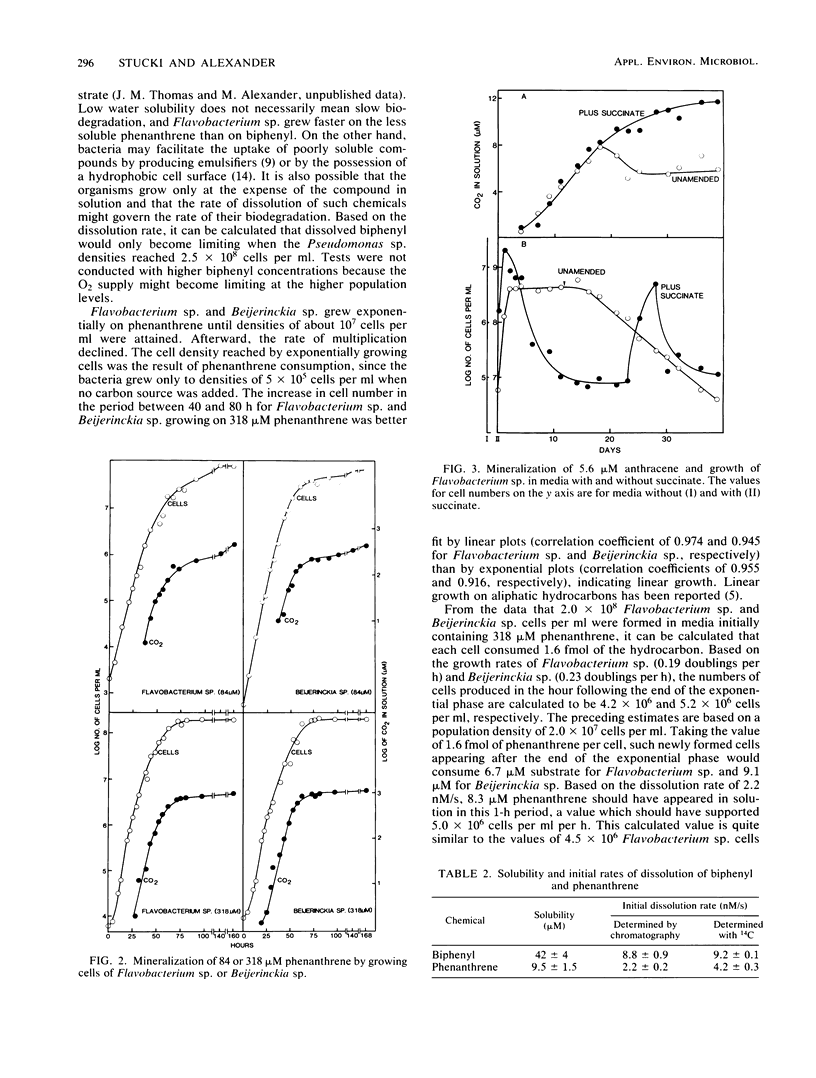

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer J. E., Capone D. G. Degradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and naphthalene in intertidal marine sediments. Appl Environ Microbiol. 1985 Jul;50(1):81–90. doi: 10.1128/aem.50.1.81-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Guerra-Santos L., Käppeli O., Fiechter A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Environ Microbiol. 1984 Aug;48(2):301–305. doi: 10.1128/aem.48.2.301-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D. L., Rosenberg E. Oil tankers and pollution: a microbiological approach. Annu Rev Microbiol. 1977;31:379–396. doi: 10.1146/annurev.mi.31.100177.002115. [DOI] [PubMed] [Google Scholar]
- Kaeppeli O., Fiechter A. The mode of interaction between the substrate and cell surface of the hydrocarbon-utilizing yeast Candida tropicalis. Biotechnol Bioeng. 1976 Jul;18(7):967–974. doi: 10.1002/bit.260180709. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käppeli O., Walther P., Mueller M., Fiechter A. Structure of the cell surface of the yeast Candida tropicalis and its relation to hydrocarbon transport. Arch Microbiol. 1984 Aug;138(4):279–282. doi: 10.1007/BF00410890. [DOI] [PubMed] [Google Scholar]
- Neufeld R. J., Zajic J. E., Gerson D. F. Cell surface measurements in hydrocarbon and carbohydrate fermentations. Appl Environ Microbiol. 1980 Mar;39(3):511–517. doi: 10.1128/aem.39.3.511-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Perry A., Gibson D. T., Gutnick D. L. Emulsifier of Arthrobacter RAG-1: specificity of hydrocarbon substrate. Appl Environ Microbiol. 1979 Mar;37(3):409–413. doi: 10.1128/aem.37.3.409-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. M., Yordy J. R., Amador J. A., Alexander M. Rates of dissolution and biodegradation of water-insoluble organic compounds. Appl Environ Microbiol. 1986 Aug;52(2):290–296. doi: 10.1128/aem.52.2.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Bertolini D. Physical state in which naphthalene and bibenzyl are utilized by bacteria. Appl Microbiol. 1972 Jun;23(6):1077–1081. doi: 10.1128/am.23.6.1077-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Coyle J. E. Physical state of phenanthrene for utilization by bacteria. Appl Microbiol. 1974 Jun;27(6):1081–1084. doi: 10.1128/am.27.6.1081-1084.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber I. K., Rosenberg E., Gutnick D. Incorporation of P and Growth of Pseudomonad UP-2 on n-Tetracosane. Appl Environ Microbiol. 1980 Dec;40(6):1086–1093. doi: 10.1128/aem.40.6.1086-1093.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


