Abstract
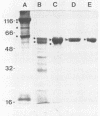
Enzymatic digestion in vitro of the Bacillus thuringiensis protoxin presumably releases and activates the toxin in a manner analogous to that which occurs when a B. thuringiensis sporulated fermentation preparation passes through the midgut of a lepidopteran larva. Therefore, a sporulated culture of B. thuringiensis subsp. kurstaki (serotype 3a3b) HD-263 was treated with trypsin to release an activated toxin soluble in bicarbonate buffer. A 63-kilodalton protein, toxic to cabbage looper larvae (Trichoplusia ni) and to lepidopteran cells in culture, was purified to homogeneity from this trypsin digest. The larvicide, a glycoprotein containing 5% carbohydrate (wt/wt), was purified from the soluble B. thuringiensis trypsin digest by using ammonium sulfate precipitation, anion-exchange chromatography, and hydrophobic-interaction chromatography. Its amino acid composition was high in nonpolar residues and unusually low in lysine and histidine. The isoelectric point was 6.5, and the amino acid on the N terminus was identified as isoleucine. The toxin was only slightly soluble in aqueous buffers unless the chaotropic agent potassium thiocyanate was added. Partial characterization of the toxin indicated that it corresponds well with reported sequences deduced from cloned genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. E., Jr, Bibilos M. M., Bulla L. A., Jr Protease activation of the entomocidal protoxin of Bacillus thuringiensis subsp. kurstaki. Appl Environ Microbiol. 1985 Oct;50(4):737–742. doi: 10.1128/aem.50.4.737-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey K. E. Purification of a protein from Bacillus thuringiensis toxic to larvae of lepidoptera. Biochem J. 1968 Jan;106(2):445–454. doi: 10.1042/bj1060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fast P. G., Martin W. G. Bacillus thuringiensis parasporal crystal toxin: dissociation into toxic low molecular weight peptides. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1314–1320. doi: 10.1016/0006-291x(80)91617-4. [DOI] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- Hanstein W. G., Davis K. A., Hatefi Y. Water structure and the chaotropic properties of haloacetates. Arch Biochem Biophys. 1971 Dec;147(2):534–544. doi: 10.1016/0003-9861(71)90411-5. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Knowles B. H., Thomas W. E., Ellar D. J. Lectin-like binding of Bacillus thuringiensis var. kurstaki lepidopteran-specific toxin is an initial step in insecticidal action. FEBS Lett. 1984 Mar 26;168(2):197–202. doi: 10.1016/0014-5793(84)80245-8. [DOI] [PubMed] [Google Scholar]
- Malik N., Berrie A. New stain fixative for proteins separated by gel isoelectric focusing based on Coomassie Brilliant Blue. Anal Biochem. 1972 Sep;49(1):173–176. doi: 10.1016/0003-2697(72)90255-2. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Lingg A. J., Bulla L. A., Jr Bacterial, viral, and fungal insecticides. Science. 1983 Feb 11;219(4585):715–721. doi: 10.1126/science.219.4585.715. [DOI] [PubMed] [Google Scholar]
- Oudin J. Immunochemical analysis by antigen-antibody precipitation in gels. Methods Enzymol. 1980;70(A):166–198. doi: 10.1016/s0076-6879(80)70048-4. [DOI] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Salaman M. R., Williamson A. R. Isoelectric focusing of proteins in the native and denatured states. Anomalous behaviour of plasma albumin. Biochem J. 1971 Mar;122(1):93–99. doi: 10.1042/bj1220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf H. E., Wong H. C., Whiteley H. R. The amino acid sequence of a crystal protein from Bacillus thuringiensis deduced from the DNA base sequence. J Biol Chem. 1985 May 25;260(10):6264–6272. [PubMed] [Google Scholar]
- Shibano Y., Yamagata A., Nakamura N., Iizuka T., Sugisaki H., Takanami M. Nucleotide sequence coding for the insecticidal fragment of the Bacillus thuringiensis crystal protein. Gene. 1985;34(2-3):243–251. doi: 10.1016/0378-1119(85)90133-7. [DOI] [PubMed] [Google Scholar]
- Tojo A., Aizawa K. Dissolution and Degradation of Bacillus thuringiensis delta-Endotoxin by Gut Juice Protease of the Silkworm Bombyx mori. Appl Environ Microbiol. 1983 Feb;45(2):576–580. doi: 10.1128/aem.45.2.576-580.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Iizuka T. Two types of entomocidal toxins in the parasporal crystals of Bacillus thuringiensis kurstaki. Arch Biochem Biophys. 1983 Nov;227(1):233–241. doi: 10.1016/0003-9861(83)90366-1. [DOI] [PubMed] [Google Scholar]