Abstract
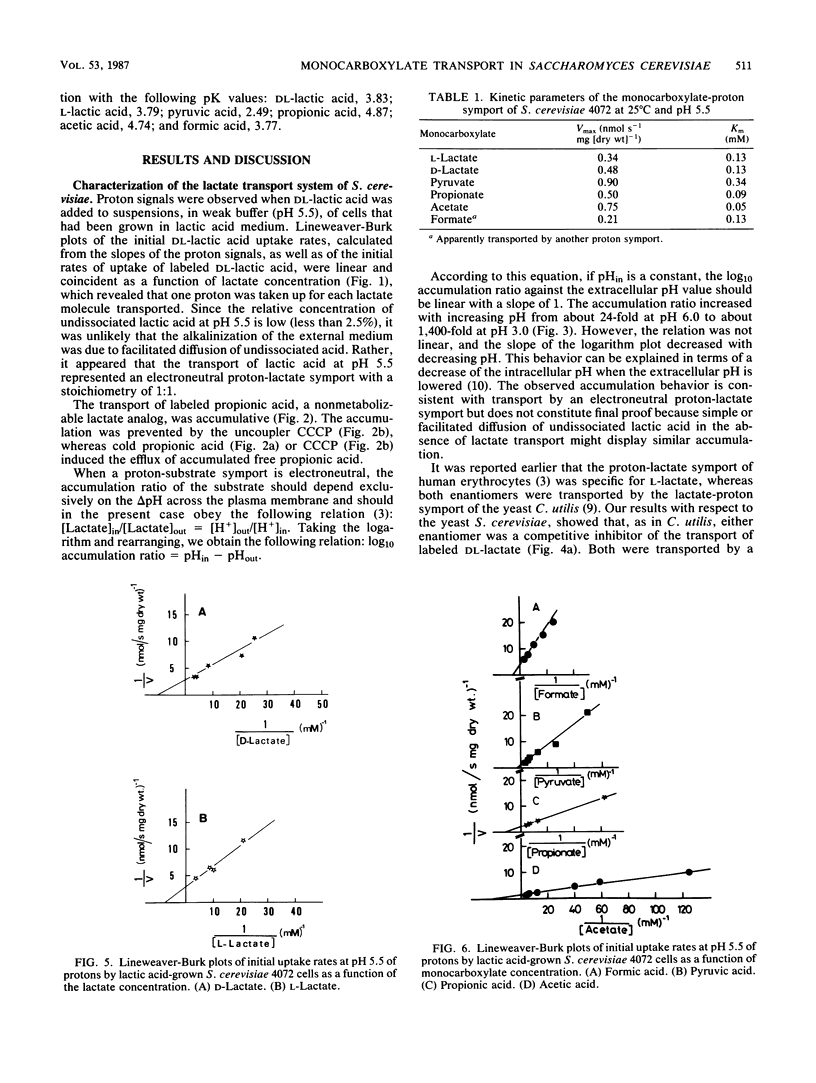
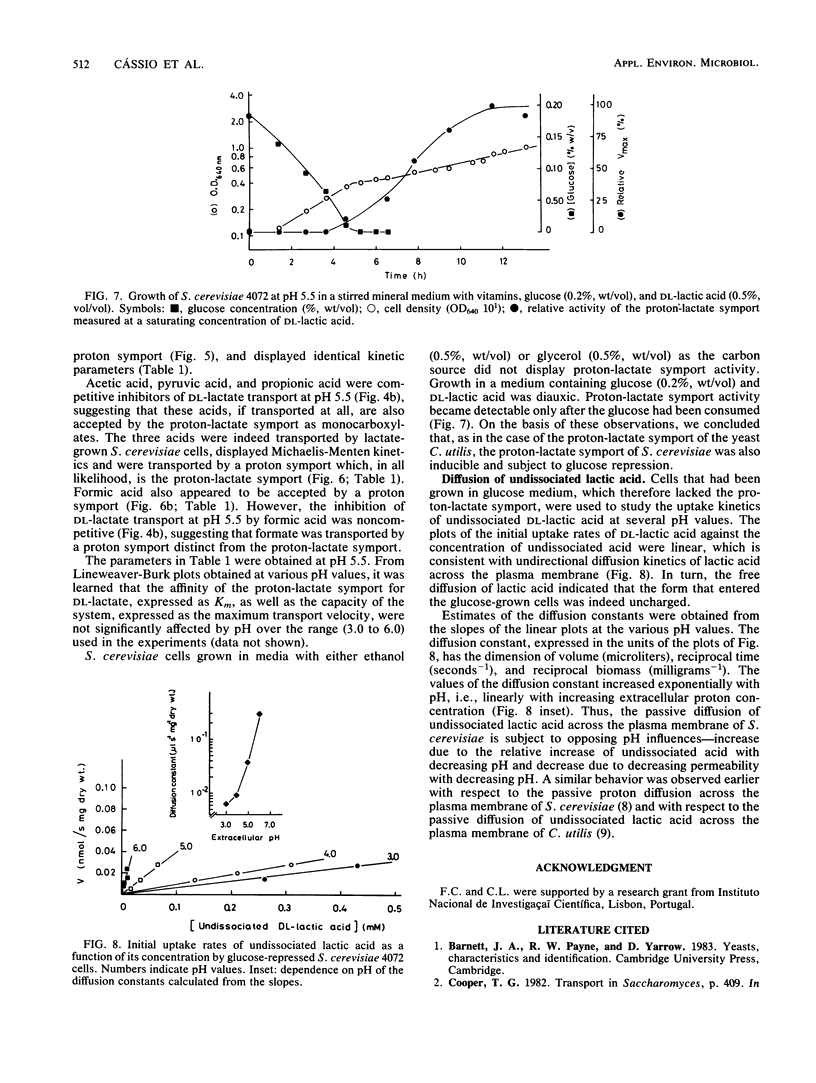
Saccharomyces cerevisiae IGC4072 grown in lactic acid medium transported lactate by an accumulative electroneutral proton-lactate symport with a proton-lactate stoichiometry of 1:1. The accumulation ratio measured with propionate increased with decreasing pH from ca. 24-fold at pH 6.0 to ca. 1,400-fold at pH 3.0. The symport accepted the following monocarboxylates (Km values at 25 degrees C and pH 5.5): D-lactate (0.13 mM), L-lactate (0.13 mM), pyruvate (0.34 mM), propionate (0.09 mM), and acetate (0.05 mM), whereas apparently a different proton symport accepted formate (0.13 mM). The lactate system was inducible and was subject to glucose repression. Undissociated lactic acid entered the cells by simple diffusion. The permeability of the plasma membrane for undissociated lactic acid increased exponentially with pH, and the diffusion constant increased 40-fold when the pH was increased from 3.0 to 6.0.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Bruijne A. W., Vreeburg H., Van Steveninck J. Kinetic analysis of L-lactate transport in human erythrocytes via the monocarboxylate-specific carrier system. Biochim Biophys Acta. 1983 Aug 10;732(3):562–568. doi: 10.1016/0005-2736(83)90232-8. [DOI] [PubMed] [Google Scholar]
- Eddy A. A., Hopkins P. G. The putative electrogenic nitrate-proton symport of the yeast Candida utilis. Comparison with the systems absorbing glucose or lactate. Biochem J. 1985 Oct 15;231(2):291–297. doi: 10.1042/bj2310291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfer M., Misra P. C. Evidence for a proton/sugar symport in the yeast Rhodotorula gracilis (glutinis). Biochem J. 1978 Apr 15;172(1):15–22. doi: 10.1042/bj1720015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão C., Van Uden N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jul 11;774(1):43–48. doi: 10.1016/0005-2736(84)90272-4. [DOI] [PubMed] [Google Scholar]
- Ruiz L. P., Gurnsey J. C., Short J. L. Reduction of Lactic Acid, Nonprotein Nitrogen, and Ash in Lactic Acid Whey by Candida ingens Culture. Appl Environ Microbiol. 1978 Apr;35(4):771–776. doi: 10.1128/aem.35.4.771-776.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña P., Barros F., Gascón S., Ramos S., Lazo P. S. The electrochemical proton gradient of Saccharomyces. The role of potassium. Eur J Biochem. 1982 Apr 1;123(2):447–453. doi: 10.1111/j.1432-1033.1982.tb19788.x. [DOI] [PubMed] [Google Scholar]
- van Uden N. Transport-limited fermentation and growth of saccharomyces cerevisiae and its competitive inhibition. Arch Mikrobiol. 1967;58(2):155–168. doi: 10.1007/BF00406676. [DOI] [PubMed] [Google Scholar]


