Abstract
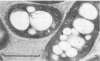
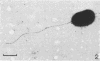
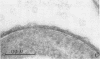
Several sulfate-reducing microorganisms were isolated from an anaerobic-purification plant. Four strains were classified as Desulfovibrio desulfuricans, Desulfovibrio sapovorans, Desulfobulbus propionicus, and Desulfovibrio sp. The D. sapovorans strain contained poly-β-hydroxybutyrate granules and seemed to form extracellular vesicles. A fifth isolate, Desulfovibrio sp. strain EDK82, was a gram-negative, non-spore-forming, nonmotile, curved organism. It was able to oxidize several substrates, including methanol. Sulfate, sulfite, thiosulfate, and sulfur were utilized as electron acceptors. Pyruvate, fumarate, malate, and glycerol could be fermented. Because strain EDK82 could not be ascribed to any of the existing species, a new species, Desulfovibrio carbinolicus, is proposed. The doubling times of the isolates were determined on several substrates. Molecular hydrogen, lactate, propionate, and ethanol yielded the shortest doubling times (3.0 to 6.3 h). Due to the presence of support material in an anaerobic filter system, these species were able to convert sulfate to sulfide very effectively at a hydraulic retention time as short as 0.5 h.
Full text
PDF


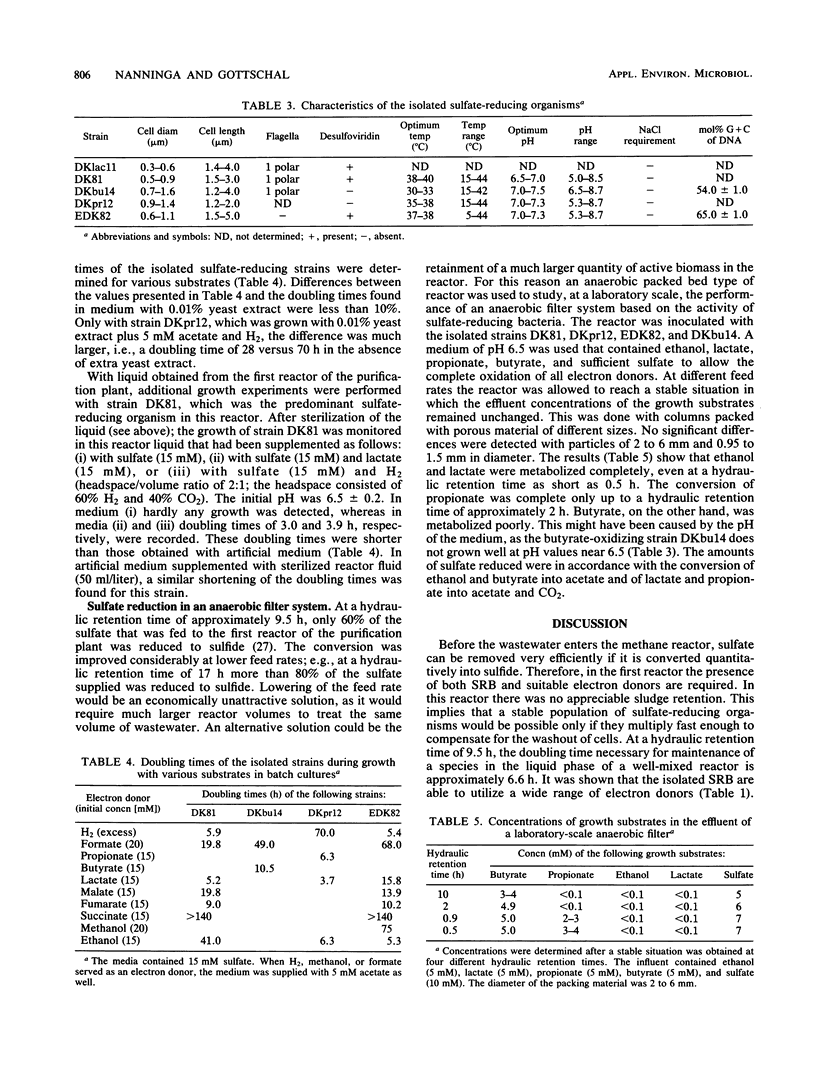



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biebl H., Pfennig Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch Microbiol. 1977 Feb 4;112(1):115–117. doi: 10.1007/BF00446664. [DOI] [PubMed] [Google Scholar]
- Campbell L. L., Kasprzycki M. A., Postgate J. R. Desulfovibrio Africans sp. n., a new dissimilatory sulfate-reducing bacterium. J Bacteriol. 1966 Oct;92(4):1122–1127. doi: 10.1128/jb.92.4.1122-1127.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa Z., Grusenmeyer S., Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: technical aspects. Appl Environ Microbiol. 1986 Mar;51(3):572–579. doi: 10.1128/aem.51.3.572-579.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanbroek H. J., Geerligs H. J., Sijtsma L., Veldkamp H. Competition for sulfate and ethanol among desulfobacter, desulfobulbus, and desulfovibrio species isolated from intertidal sediments. Appl Environ Microbiol. 1984 Feb;47(2):329–334. doi: 10.1128/aem.47.2.329-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Effect of inorganic sulfide on the growth and metabolism of Methanosarcina barkeri strain DM. Appl Environ Microbiol. 1979 Apr;37(4):670–675. doi: 10.1128/aem.37.4.670-675.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga H. J., Gottschal J. C. Microbial problems with waste from potato-starch processing. Microbiol Sci. 1986 Jun;3(6):179–182. [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- Schoberth S. A new strain of Desulfovibrio gigas isolated from a sewage plant. Arch Mikrobiol. 1973;92(4):365–368. doi: 10.1007/BF00409290. [DOI] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Thauer R. K. Dissimilatory sulphate reduction with acetate as electron donor. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):467–471. doi: 10.1098/rstb.1982.0092. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]