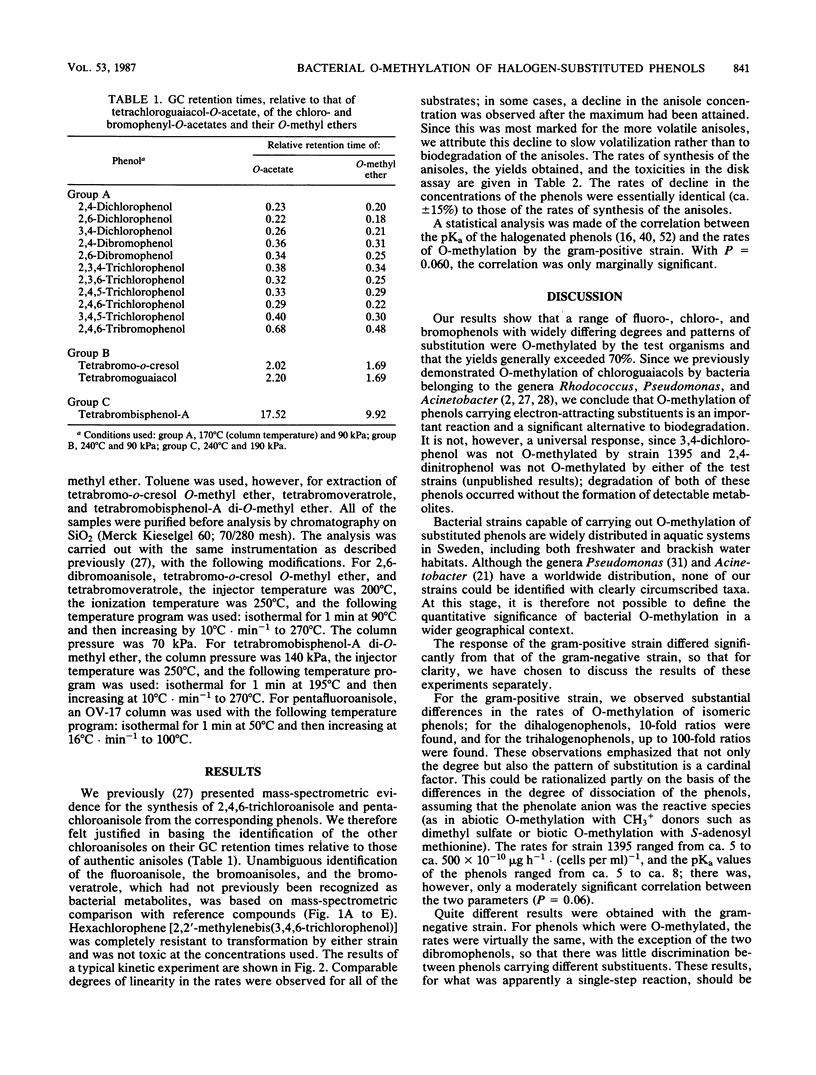
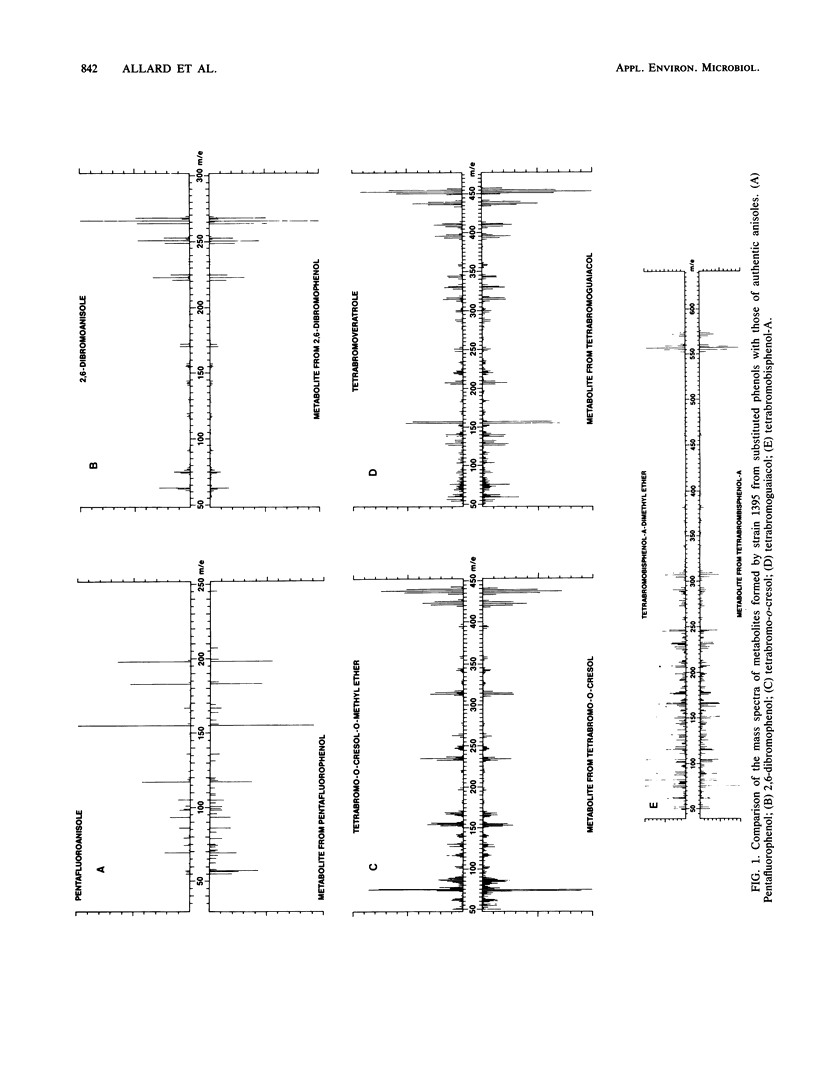
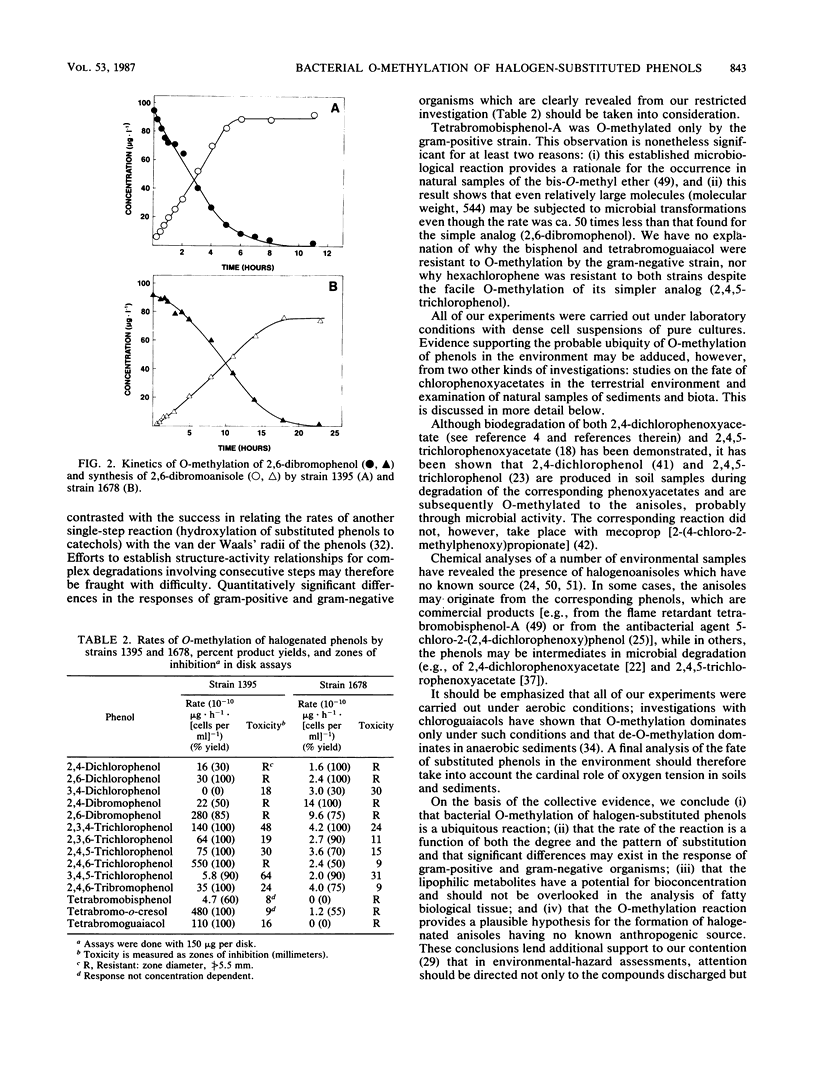
Abstract
Two strains of bacteria capable of carrying out the O-methylation of phenolic compounds, one from the gram-positive genus Rhodococcus and one from the gram-negative genus Acinetobacter, were used to examine the O-methylation of phenols carrying fluoro-, chloro-, and bromo-substituents. Zero-order rates of O-methylation were calculated from data for the chloro- and bromophenols; there was no simple relationship between the rates of reaction and the structure of the substrates, and significant differences were observed in the responses of the two test organisms. For the gram-negative strain, the pattern of substitution was as important as the number of substituents. Hexachlorophene was resistant to O-methylation by both strains, and tetrabromobisphenol-A was O-methylated only by the gram-positive strain. It is suggested that in the natural environment, bacterial O-methylation of phenols carrying electron-attracting substituents might be a significant alternative to biodegradation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg U. G., Thunberg T. M. Chlorinated phenols: occurrence, toxicity, metabolism, and environmental impact. Crit Rev Toxicol. 1980 Jul;7(1):1–35. doi: 10.3109/10408448009017934. [DOI] [PubMed] [Google Scholar]
- Allard A. S., Remberger M., Neilson A. H. Bacterial o-methylation of chloroguaiacols: effect of substrate concentration, cell density, and growth conditions. Appl Environ Microbiol. 1985 Feb;49(2):279–288. doi: 10.1128/aem.49.2.279-288.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Schulke J. W., Frazier L. M., Seidler R. J. Characterization of aquatic bacteria and cloning of genes specifying partial degradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1985 May;49(5):1237–1245. doi: 10.1128/aem.49.5.1237-1245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. P., Kirsch E. J. Metabolism of pentachlorophenol by an axenic bacterial culture. Appl Microbiol. 1972 May;23(5):1033–1035. doi: 10.1128/am.23.5.1033-1035.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi A. J., Johnson E. L. Methylation of pentachlorophenol by Trichoderma virgatum. Can J Microbiol. 1972 Jan;18(1):45–49. doi: 10.1139/m72-007. [DOI] [PubMed] [Google Scholar]
- Di Carlo F. J., Seifter J., DeCarlo V. J. Assessment of the hazards of polybrominated biphenyls. Environ Health Perspect. 1978 Apr;23:351–365. doi: 10.1289/ehp.7823351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. M., Peel J. L. Metabolism of 2,3,4,6-tetrachlorophenol by micro-organisms from broiler house litter. J Gen Microbiol. 1974 Dec;85(2):237–243. doi: 10.1099/00221287-85-2-237. [DOI] [PubMed] [Google Scholar]
- Hunt S., Breuer S. W. Isolation of a new naturally occurring halogenated amino acid: monochloromonobromotyrosine. Biochim Biophys Acta. 1971 Nov 12;252(2):401–404. doi: 10.1016/0304-4165(71)90021-3. [DOI] [PubMed] [Google Scholar]
- Jacobs L. W., Chou S. F., Tiedje J. M. Field concentrations and persistence of polybrominated biphenyls in soils and solubility of PBB in natural waters. Environ Health Perspect. 1978 Apr;23:1–8. doi: 10.1289/ehp.78231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P. A. Potentiometric titration of ionizable compounds in two-phase systems. IV. The extraction behaviour of halogen -- and/or nitro-substituted phenols as acids and ion pairs. Acta Pharm Suec. 1977;14(4):345–362. [PubMed] [Google Scholar]
- Kilbane J. J., Chatterjee D. K., Karns J. S., Kellogg S. T., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Jul;44(1):72–78. doi: 10.1128/aem.44.1.72-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Maier W. J. Kinetics of microbial growth on pentachlorophenol. Appl Environ Microbiol. 1985 Jan;49(1):46–53. doi: 10.1128/aem.49.1.46-53.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M. A., Roberts R. N., Alexander M. Formation of 2,4-dichlorophenol and 2,4-dichloroanisole from 2,4-dichlorophen-oxyacetate by Arthrobacter sp. Can J Microbiol. 1967 Jun;13(6):691–699. doi: 10.1139/m67-091. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Kaneko S., Horii S., Yamagishi T. Identification of polyhalogenated anisoles and phenols in oysters collected from Tokyo Bay. Bull Environ Contam Toxicol. 1981 May;26(5):577–584. doi: 10.1007/BF01622140. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Yamagishi T., Matsumoto M. Residues of 4-chloro-1-(2,4-dichlorophenoxy)-2-methoxybenzene(triclosan methyl) in aquatic biota. Bull Environ Contam Toxicol. 1984 Feb;32(2):227–232. doi: 10.1007/BF01607490. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Hynning P. A., Remberger M., Landner L. Bacterial methylation of chlorinated phenols and guaiacols: formation of veratroles from guaiacols and high-molecular-weight chlorinated lignin. Appl Environ Microbiol. 1983 Mar;45(3):774–783. doi: 10.1128/aem.45.3.774-783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C. Structure-activity relationships in microbial transformation of phenols. Appl Environ Microbiol. 1982 Jul;44(1):153–158. doi: 10.1128/aem.44.1.153-158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatello J. J., Johnson L. K., Martinson M. M., Carlson R. E., Crawford R. L. Response of the microflora in outdoor experimental streams to pentachlorophenol: compartmental contributions. Appl Environ Microbiol. 1985 Jul;50(1):127–132. doi: 10.1128/aem.50.1.127-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remberger M., Allard A. S., Neilson A. H. Biotransformations of chloroguaiacols, chlorocatechols, and chloroveratroles in sediments. Appl Environ Microbiol. 1986 Mar;51(3):552–558. doi: 10.1128/aem.51.3.552-558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber D. L., Crawford R. L. Isolation and characterization of Flavobacterium strains that degrade pentachlorophenol. Appl Environ Microbiol. 1985 Dec;50(6):1512–1518. doi: 10.1128/aem.50.6.1512-1518.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E. Identification of 2,4-dichloroanisole and 2,4-dichlorophenol as soil degradation products of ring-labelled [14C]2,4-D. Bull Environ Contam Toxicol. 1985 Feb;34(2):150–157. doi: 10.1007/BF01609717. [DOI] [PubMed] [Google Scholar]
- Smith A. E. Identification of 4-chloro-2-methylphenol as a soil degradation product of ring-labelled [14C]mecoprop. Bull Environ Contam Toxicol. 1985 May;34(5):656–660. doi: 10.1007/BF01609789. [DOI] [PubMed] [Google Scholar]
- Stanlake G. J., Finn R. K. Isolation and characterization of a pentachlorophenol-degrading bacterium. Appl Environ Microbiol. 1982 Dec;44(6):1421–1427. doi: 10.1128/aem.44.6.1421-1427.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Metabolism of pentachlorophenol by a soil microbe. J Environ Sci Health B. 1977;12(2):113–127. doi: 10.1080/03601237709372057. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Kashimoto T., Tatsukawa R. Brominated phenols and anisoles in river and marine sediments in Japan. Bull Environ Contam Toxicol. 1985 Aug;35(2):272–278. doi: 10.1007/BF01636509. [DOI] [PubMed] [Google Scholar]


