Abstract
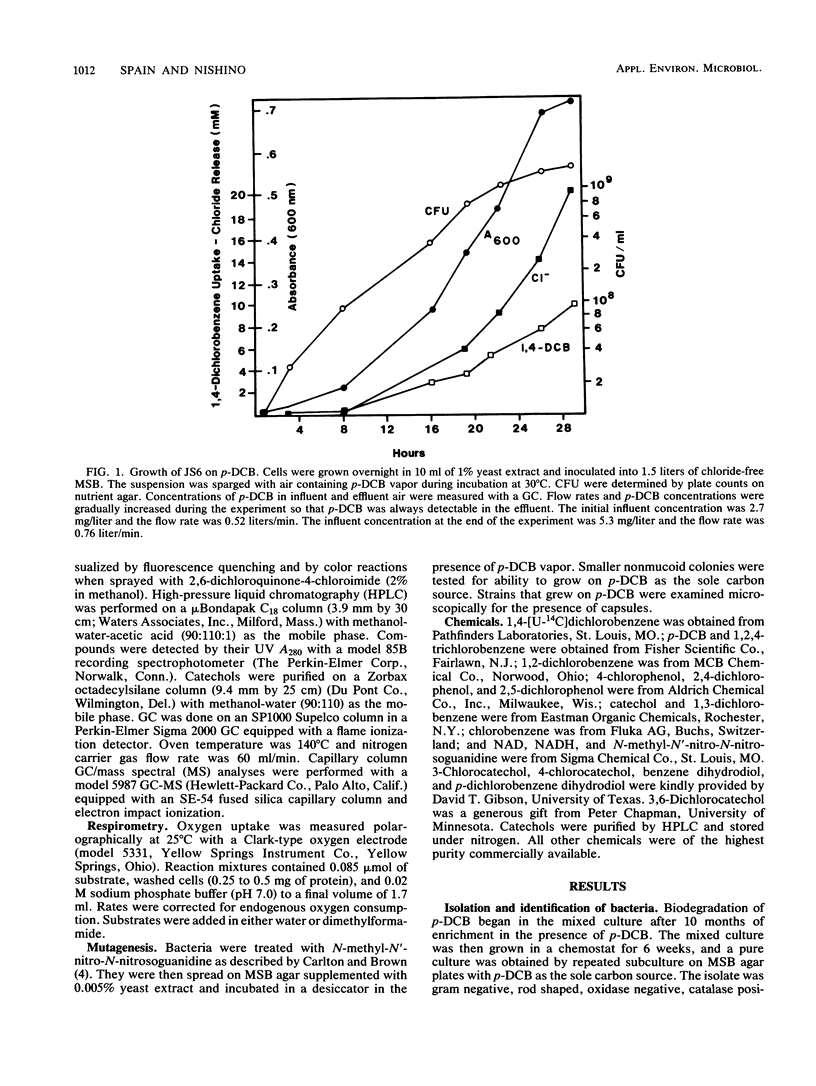
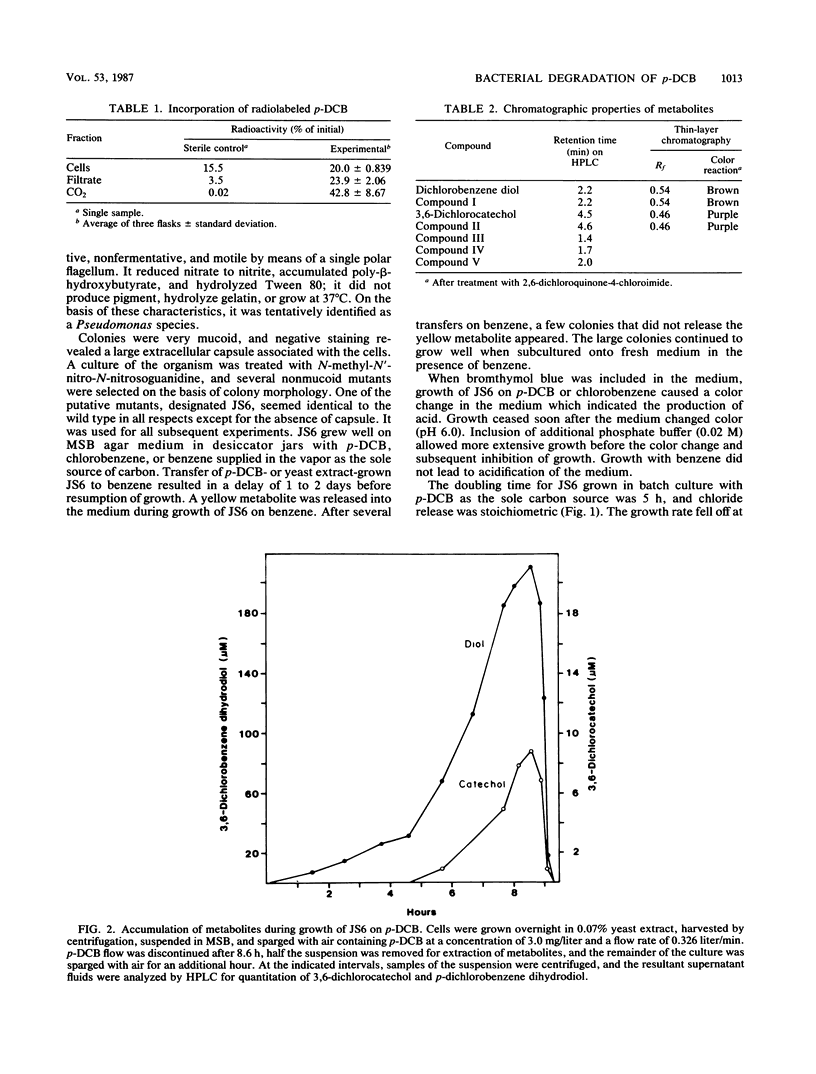
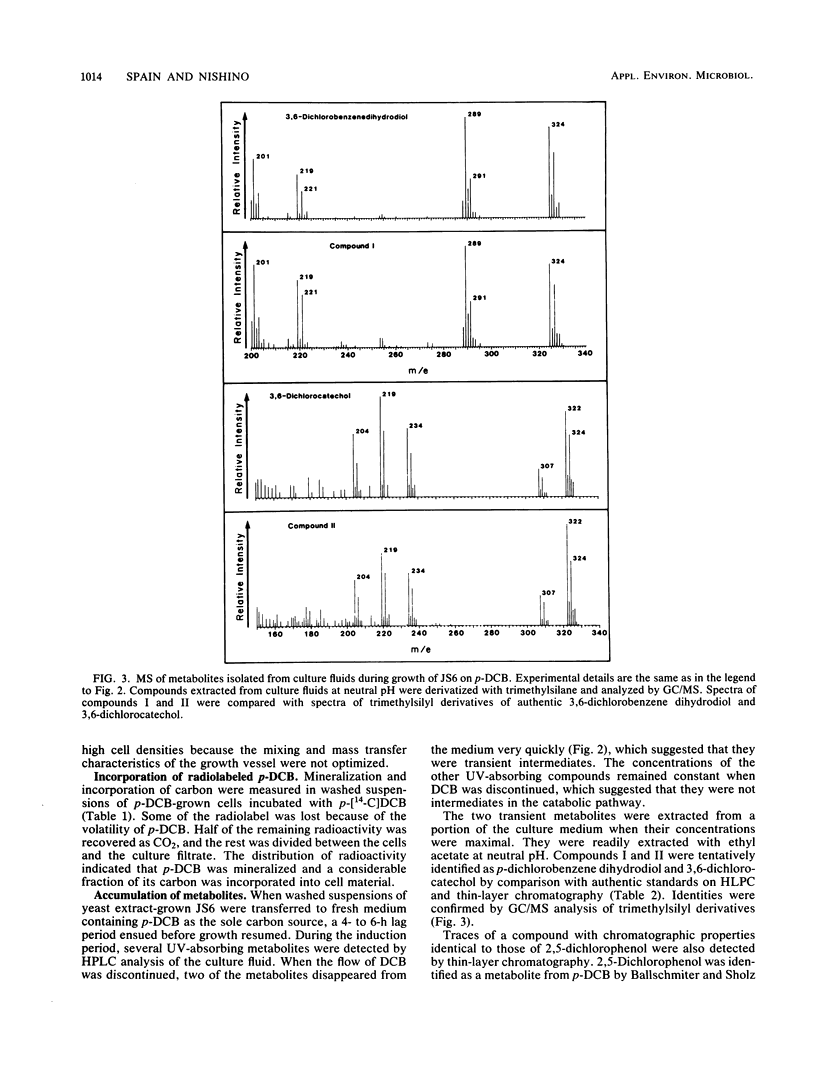
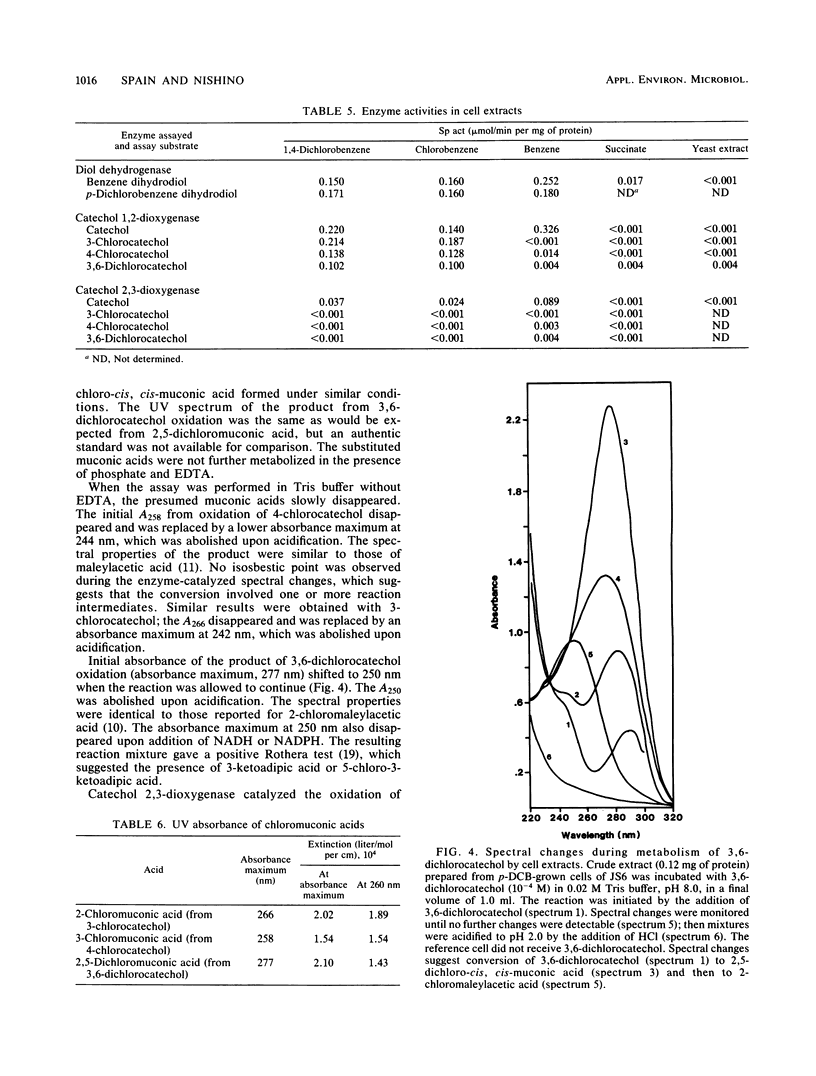
A Pseudomonas species able to degrade p-dichlorobenzene as the sole source of carbon and energy was isolated by selective enrichment from activated sludge. The organism also grew well on chlorobenzene and benzene. Washed cells released chloride in stoichiometric amounts from o-, m-, and p-dichlorobenzene, 2,5-dichlorophenol, 4-chlorophenol, 3-chlorocatechol, 4-chlorocatechol, and 3,6-dichlorocatechol. Initial steps in the pathway for p-dichlorobenzene degradation were determined by isolation of metabolites, simultaneous adaptation studies, and assay of enzymes in cell extracts. Results indicate that p-dichlorobenzene was initially converted by a dioxygenase to 3,6-dichloro-cis-1,2-dihydroxycyclohexa-3,5-diene, which was converted to 3,6-dichlorocatechol by an NAD+-dependent dehydrogenase. Ring cleavage of 3,6-dichlorocatechol was by a 1,2-oxygenase to form 2,5-dichloro-cis, cis-muconate. Enzymes for degradation of haloaromatic compounds were induced in cells grown on chlorobenzene or p-dichlorobenzene, but not in cells grown on benzene, succinate, or yeast extract. Enzymes of the ortho pathway induced in cells grown on benzene did not attack chlorobenzenes or chlorocatechols.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury J. M., Tiedje J. M., Alexander M., Dawson J. E. 2,4-D metabolism: enzymatic conversion of chloromaleylacetic acid to succinic acid. J Agric Food Chem. 1970 Mar-Apr;18(2):199–201. doi: 10.1021/jf60168a029. [DOI] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Fernley H. N., Davies J. I. Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem J. 1971 May;122(4):543–551. doi: 10.1042/bj1220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Moss P., Fernley H. N. Bacterial metabolism of 4-chlorophenoxyacetate. Biochem J. 1971 May;122(4):509–517. doi: 10.1042/bj1220509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins S. R., Tomson M. B., Wilson J. T., Ward C. H. Microbial removal of wastewater organic compounds as a function of input concentration in soil columns. Appl Environ Microbiol. 1984 Nov;48(5):1039–1045. doi: 10.1128/aem.48.5.1039-1045.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILBY B. A. The formation of beta-ketoadipic acid by bacterial fission of aromatic rings. Biochem J. 1951 Oct;49(5):671–674. doi: 10.1042/bj0490671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg S. T., Chatterjee D. K., Chakrabarty A. M. Plasmid-assisted molecular breeding: new technique for enhanced biodegradation of persistent toxic chemicals. Science. 1981 Dec 4;214(4525):1133–1135. doi: 10.1126/science.7302584. [DOI] [PubMed] [Google Scholar]
- Klages U., Markus A., Lingens F. Degradation of 4-chlorophenylacetic acid by a Pseudomonas species. J Bacteriol. 1981 Apr;146(1):64–68. doi: 10.1128/jb.146.1.64-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Inhibition of catechol 2,3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol. 1981 May;41(5):1159–1165. doi: 10.1128/aem.41.5.1159-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks T. S., Wait R., Smith A. R., Quirk A. V. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem Biophys Res Commun. 1984 Oct 30;124(2):669–674. doi: 10.1016/0006-291x(84)91607-3. [DOI] [PubMed] [Google Scholar]
- Markus A., Klages U., Lingens F. Mikrobieller Abbau von 4-Chlorphenylessigsäure. Chemische Synthese von 3-Chlor-4-hydroxy-, 4-Chlor-3-hydroxy- und 4-Chlor-2-hydroxyphenylessigsäure. Hoppe Seylers Z Physiol Chem. 1982 Apr;363(4):431–437. [PubMed] [Google Scholar]
- Reineke W., Jeenes D. J., Williams P. A., Knackmuss H. J. TOL plasmid pWW0 in constructed halobenzoate-degrading Pseudomonas strains: prevention of meta pathway. J Bacteriol. 1982 Apr;150(1):195–201. doi: 10.1128/jb.150.1.195-201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Hybrid pathway for chlorobenzoate metabolism in Pseudomonas sp. B13 derivatives. J Bacteriol. 1980 May;142(2):467–473. doi: 10.1128/jb.142.2.467-473.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol. 1984 Feb;47(2):395–402. doi: 10.1128/aem.47.2.395-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraa G., Boone M. L., Jetten M. S., van Neerven A. R., Colberg P. J., Zehnder A. J. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Appl Environ Microbiol. 1986 Dec;52(6):1374–1381. doi: 10.1128/aem.52.6.1374-1381.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bont J. A., Vorage M. J., Hartmans S., van den Tweel W. J. Microbial degradation of 1,3-dichlorobenzene. Appl Environ Microbiol. 1986 Oct;52(4):677–680. doi: 10.1128/aem.52.4.677-680.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


