Abstract
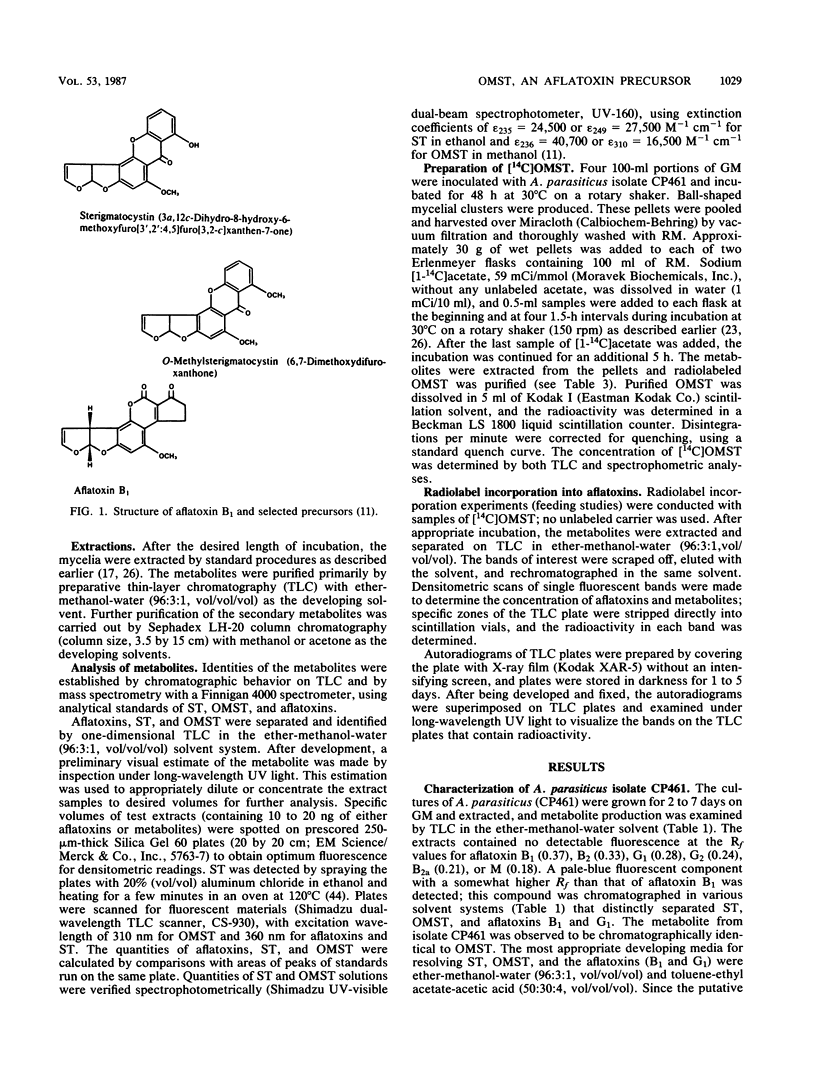
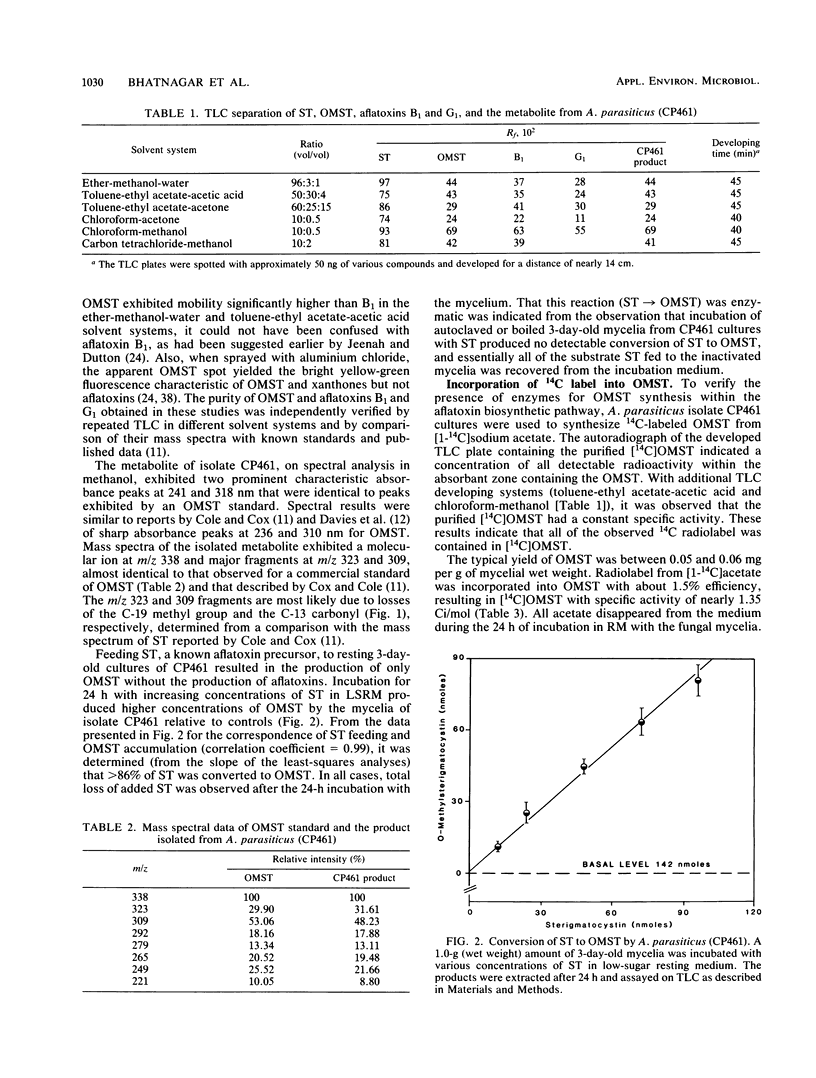
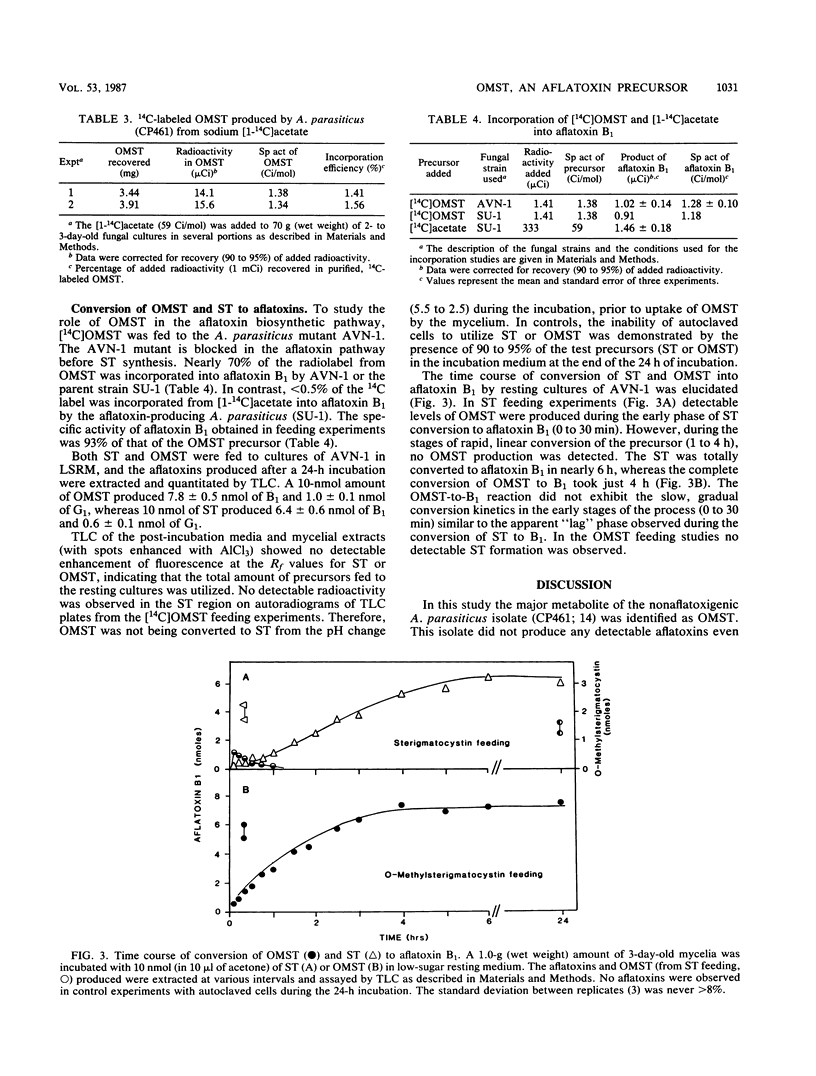
An isolate of Aspergillus parasiticus CP461 (SRRC 2043) produced no detectable aflatoxins, but accumulated O-methylsterigmatocystin (OMST). When sterigmatocystin (ST) was fed to this isolate in a low-sugar medium, there was an increase in the accumulation of OMST, without aflatoxin synthesis. When radiolabeled [14C]OMST was fed to resting mycelia of a non-aflatoxin-, non-ST-, and non-OMST-producing mutant of A. parasiticus AVN-1 (SRRC 163), 14C-labeled aflatoxins B1 and G1 were produced; 10 nmol of OMST produced 7.8 nmol of B1 and 1.0 nmol of G1, while 10 nmol of ST produced 6.4 nmol of B1 and 0.6 nmol of G1. A time course study of aflatoxin synthesis in ST feeding experiments with AVN-1 revealed that OMST is synthesized by the mold during the onset of aflatoxin synthesis. The total amount of aflatoxins recovered from OMST feeding experiments was higher than from experiments in which ST was fed to the resting mycelia. These results suggest that OMST is a true metabolite in the aflatoxin biosynthetic pathway between sterigmatocystin and aflatoxins B1 and G1 and is not a shunt metabolite, as thought previously.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J., MATELES R. I. INCORPORATION OF LABELLED COMPOUNDS INTO AFLATOXINS. Biochim Biophys Acta. 1964 May 11;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- Anderson M. S., Dutton M. F. The use of cell free extracts derived from fungal protoplasts in the study of aflatoxin biosynthesis. Experientia. 1979 Jan 15;35(1):21–22. doi: 10.1007/BF01917850. [DOI] [PubMed] [Google Scholar]
- Ayer W. A., Pena-Rodriguez L., Vederas J. C. Identification of sterigmatocystin as a metabolite of Monocillium nordinii. Can J Microbiol. 1981 Aug;27(8):846–847. doi: 10.1139/m81-131. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Christensen S. B. New perspectives on aflatoxin biosynthesis. Adv Appl Microbiol. 1983;29:53–92. doi: 10.1016/s0065-2164(08)70354-x. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Gaar G. G. Effect of acetone on production of aflatoxins and versicolorin pigments by resting cell cultures of Aspergillus parasiticus. Mycopathologia. 1976 Jun 4;58(1):9–12. doi: 10.1007/BF00493586. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Shoss S. M., Boudreaux G. H. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl Environ Microbiol. 1980 Apr;39(4):835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biollaz M., Büchi G., Milne G. The biosynthesis of the aflatoxins. J Am Chem Soc. 1970 Feb 25;92(4):1035–1043. doi: 10.1021/ja00707a050. [DOI] [PubMed] [Google Scholar]
- Dorner J. W., Cole R. J., Diener U. L. The relationship of Aspergillus flavus and Aspergillus parasiticus with reference to production of aflatoxins and cyclopiazonic acid. Mycopathologia. 1984 Aug 30;87(1-2):13–15. doi: 10.1007/BF00436617. [DOI] [PubMed] [Google Scholar]
- Dutton M. F., Anderson M. S. Role of versicolorin A and its derivatives in aflatoxin biosynthesis. Appl Environ Microbiol. 1982 Mar;43(3):548–551. doi: 10.1128/aem.43.3.548-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton M. F., Anderson M. S. The use of fungal protoplasts in the study of aflatoxin biosynthesis. Experientia. 1978 Jan 15;34(1):22–24. doi: 10.1007/BF01921878. [DOI] [PubMed] [Google Scholar]
- Dutton M. F., Ehrlich K., Bennett J. W. Biosynthetic relationship among aflatoxins B1, B2, M1, and M2. Appl Environ Microbiol. 1985 Jun;49(6):1392–1395. doi: 10.1128/aem.49.6.1392-1395.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C. Conversion of sterigmatocystin to aflatoxin B 1 by Aspergillus parasiticus. Biochem Biophys Res Commun. 1973 Jun 8;52(3):992–997. doi: 10.1016/0006-291x(73)91035-8. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Mateles R. I. The relative contribution of acetate and glucose to aflatoxin biosynthesis. Biochim Biophys Acta. 1970 Jun;208(3):482–486. doi: 10.1016/0304-4165(70)90222-9. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Yang S. L. Preparation of 14C-labeled sterigmatocystin in liquid media. Appl Microbiol. 1975 Jan;29(1):17–20. doi: 10.1128/am.29.1.17-20.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeenah M. S., Dutton M. F. The conversion of sterigmatocystin to O-methylsterigmatocystin and aflatoxin B1 by a cell-free preparation. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1114–1118. doi: 10.1016/s0006-291x(83)80257-5. [DOI] [PubMed] [Google Scholar]
- Lafont P., Debeaupuis J. P. Effet de la sterigmatocystine sur la toxinogenese d'Aspergillus du groupe flavus. Mycopathologia. 1979 Dec 28;69(3):187–192. doi: 10.1007/BF00452833. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Ory R. L. Biosynthesis of aflatoxin B1. Conversion of versicolorin A to aflatoxin B1 by Aspergillus parasiticus. J Agric Food Chem. 1976 Nov-Dec;24(6):1167–1170. doi: 10.1021/jf60208a017. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Stanley J. B. Synthesis of versicolorin A by a mutant strain of Aspergillus parasiticus deficient in aflatoxin production. J Agric Food Chem. 1975 Nov-Dec;23(6):1132–1134. doi: 10.1021/jf60202a011. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Hsieh D. P., Yao R. C., Donkersloot J. A. Conversion of averufin into aflatoxins by Aspergillus parasiticus. Biochemistry. 1973 Dec 4;12(25):5167–5171. doi: 10.1021/bi00749a023. [DOI] [PubMed] [Google Scholar]
- Maggon K. K., Gupta S. K., Venkitasubramanian T. A. Biosynthesis of aflatoxins. Bacteriol Rev. 1977 Dec;41(4):822–855. doi: 10.1128/br.41.4.822-855.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa K. E. Genetics of Aspergillus flavus: complementation and mapping of aflatoxin mutants. Genet Res. 1979 Aug;34(1):1–9. doi: 10.1017/s0016672300019236. [DOI] [PubMed] [Google Scholar]
- Papa K. E. Genetics of Aspergillus flavus: linkage of aflatoxin mutants. Can J Microbiol. 1984 Jan;30(1):68–73. doi: 10.1139/m84-012. [DOI] [PubMed] [Google Scholar]
- Raj H. G., Viswanathan L., Murthy H. S., Venkitasubramanian T. A. Biosynthesis of aflatoxins by cell-free preparations from Aspergillus flavus. Experientia. 1969 Nov 15;25(11):1141–1142. doi: 10.1007/BF01900235. [DOI] [PubMed] [Google Scholar]
- Sekita S., Yoshihira K., Natori S., Udagawa S., Muroi T., Sugiyama Y., Kurata H., Umeda M. Mycotoxin production by Chaetomium spp. and related fungi. Can J Microbiol. 1981 Aug;27(8):766–772. doi: 10.1139/m81-119. [DOI] [PubMed] [Google Scholar]
- Shantha T., Murthy V. S. Influence of tricarboxylic acid cycle intermediates and related metabolites on the biosynthesis of aflatoxin by resting cells of Aspergillus flavus. Appl Environ Microbiol. 1981 Nov;42(5):758–761. doi: 10.1128/aem.42.5.758-761.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Aflatoxin biosynthetic pathway: elucidation by using blocked mutants of Aspergillus parasiticus. Arch Biochem Biophys. 1977 Jan 15;178(1):285–292. doi: 10.1016/0003-9861(77)90193-x. [DOI] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Enzymatic conversion of sterigmatocystin into aflatoxin B1 by cell-free extracts of Aspergillus parasiticus. Appl Environ Microbiol. 1976 May;31(5):743–745. doi: 10.1128/aem.31.5.743-745.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack M., Rodricks J. V. Method for analysis and chemical confirmation of sterigmatocystin. J Assoc Off Anal Chem. 1971 Jan;54(1):86–90. [PubMed] [Google Scholar]
- Udagawa S., Muroi T., Kurata H., Sekita S., Yoshihira K., Natori S., Umeda M. The production of chaetoglobosins, sterigmatocystin, O-methylsterigmatocystin, and chaetocin by Chaetomium spp. and related fungi. Can J Microbiol. 1979 Feb;25(2):170–177. doi: 10.1139/m79-027. [DOI] [PubMed] [Google Scholar]
- Venkitasubramanian T. A., Gupta S. K. Biosynthesis of aflatoxins. Ann Nutr Aliment. 1977;31(4-6):635–642. [PubMed] [Google Scholar]
- Wan N. C., Hsieh D. P. Enzymatic formation of the bisfuran structure in aflatoxin biosynthesis. Appl Environ Microbiol. 1980 Jan;39(1):109–112. doi: 10.1128/aem.39.1.109-112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir L. O., Ginsburg R. Aflatoxin biosynthesis: detection of transient, acetate-dependent intermediates in Aspergillus by kinetic pulse-labeling. J Bacteriol. 1979 Jun;138(3):684–690. doi: 10.1128/jb.138.3.684-690.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir L. O., Hufford K. D. Precursor recognition by kinetic pulse-labeling in a toxigenic aflatoxin B1-producing strain of Aspergillus. Appl Environ Microbiol. 1981 Jul;42(1):168–173. doi: 10.1128/aem.42.1.168-173.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Egmond H. P., Paulsch W. E., Deijll E., Schuller P. L. Thin layer chromatographic method for analysis and chemical confirmation of sterigmatocystin in cheese. J Assoc Off Anal Chem. 1980 Jan;63(1):110–114. [PubMed] [Google Scholar]


