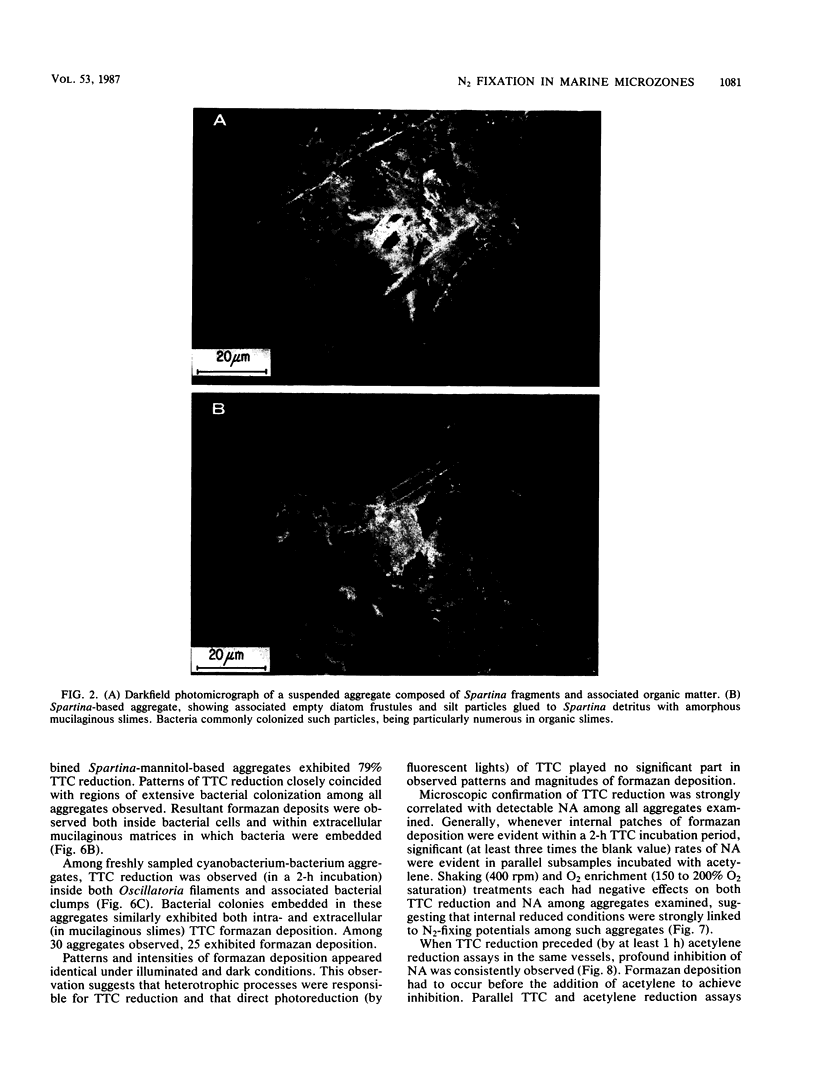
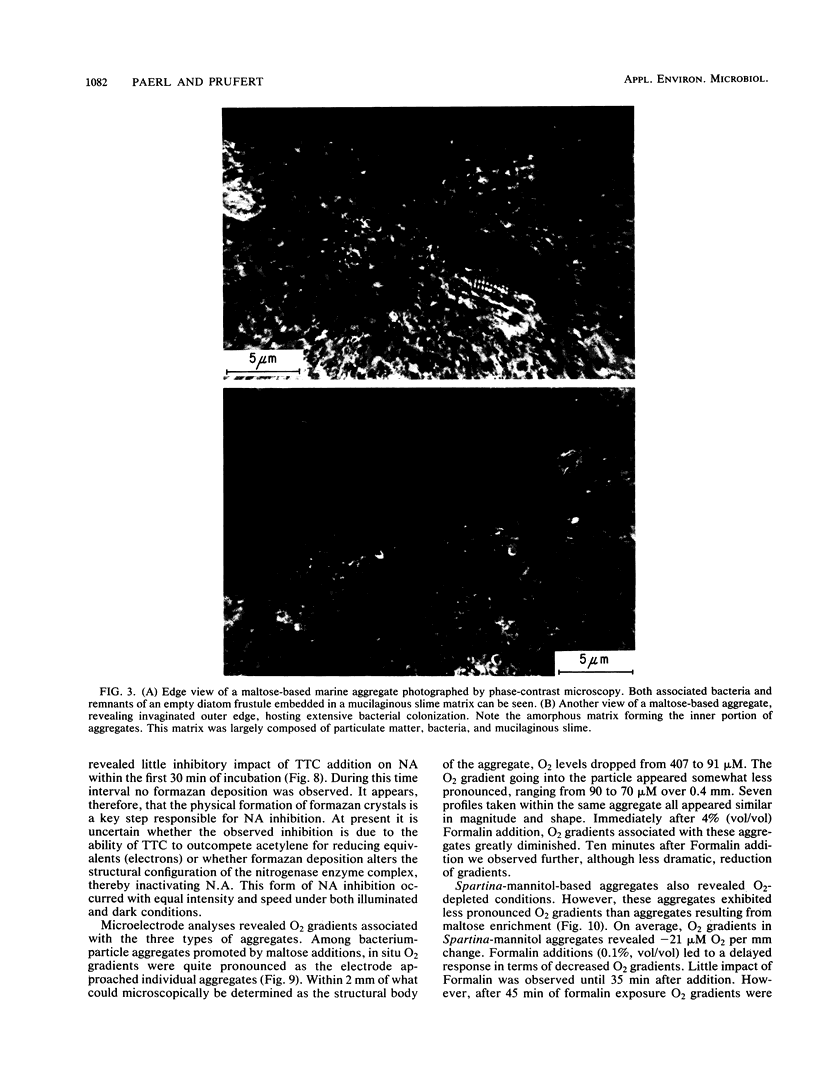
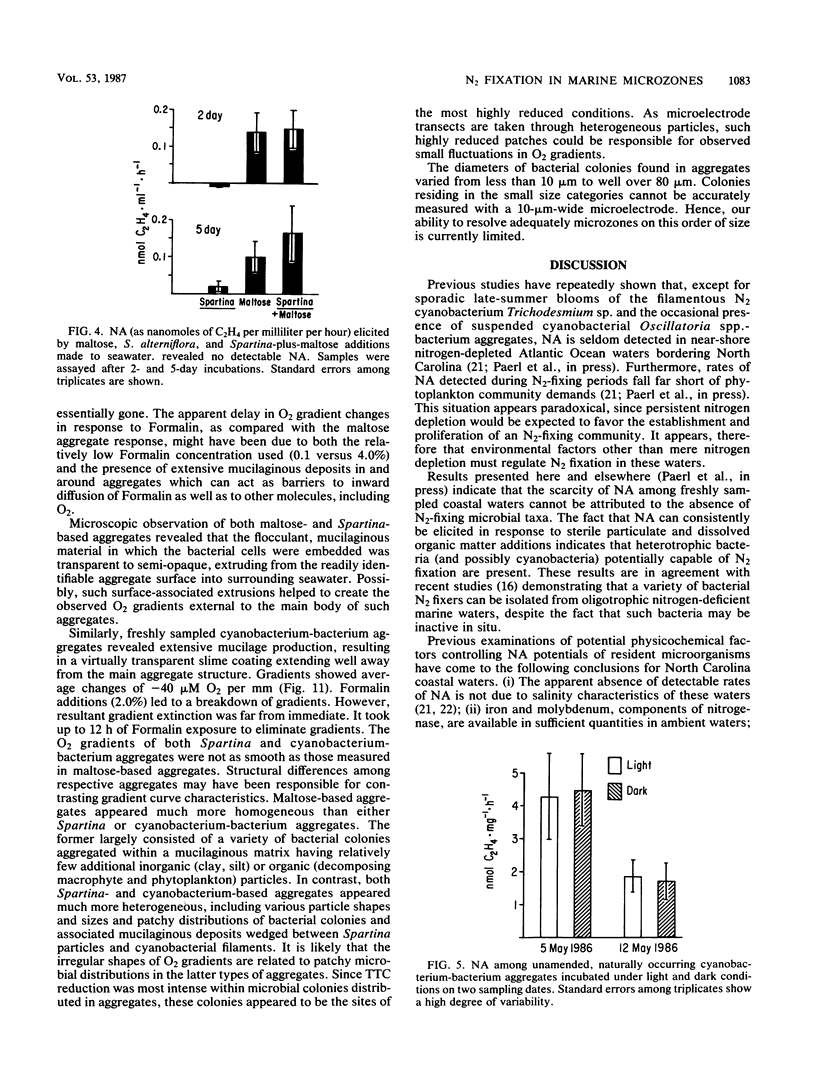
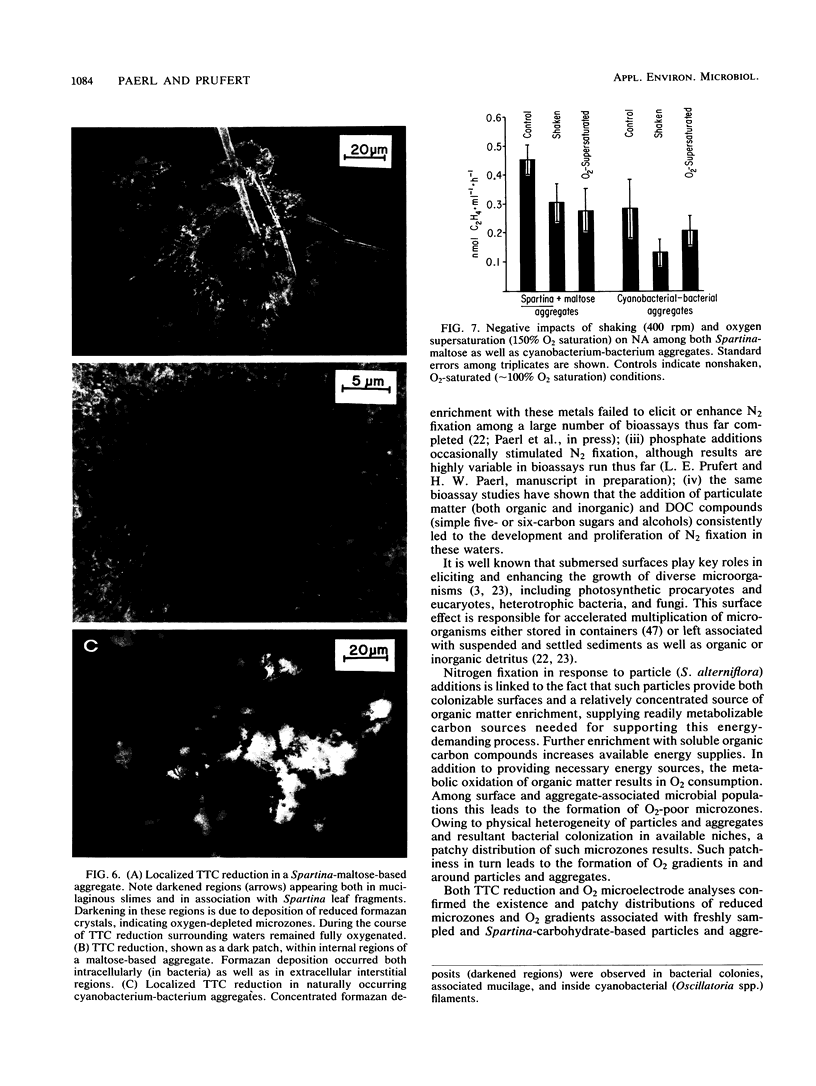
Abstract
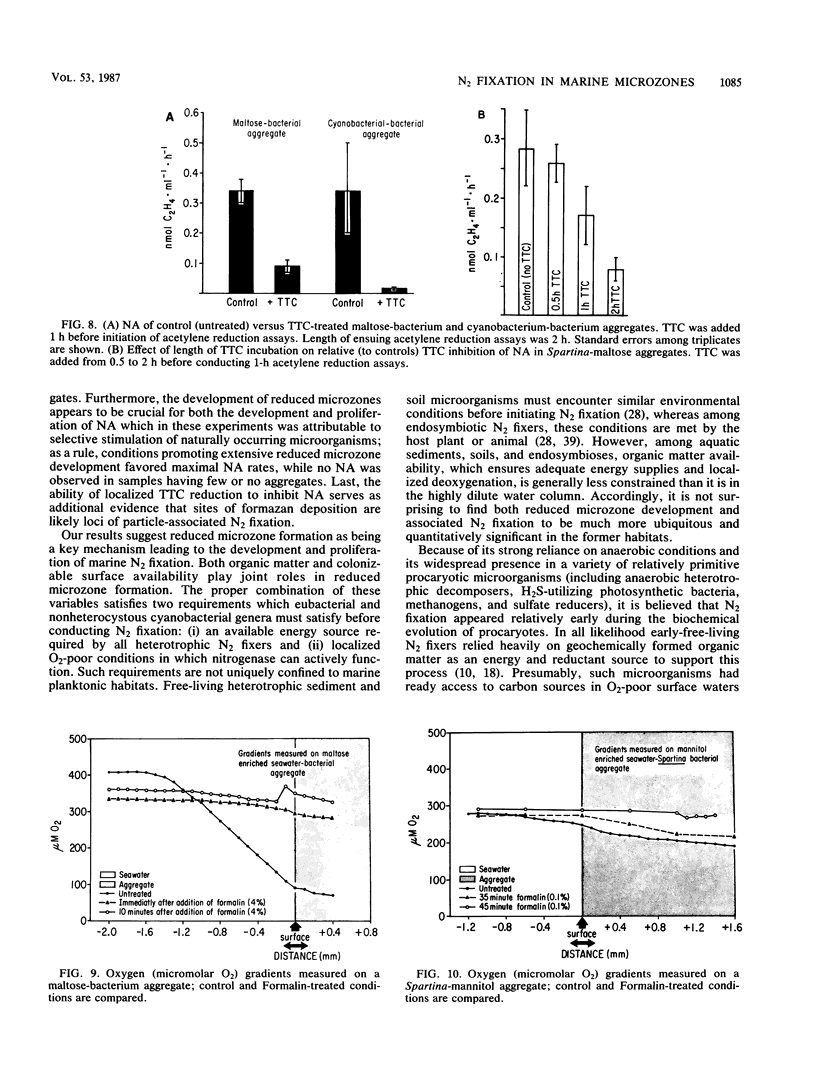
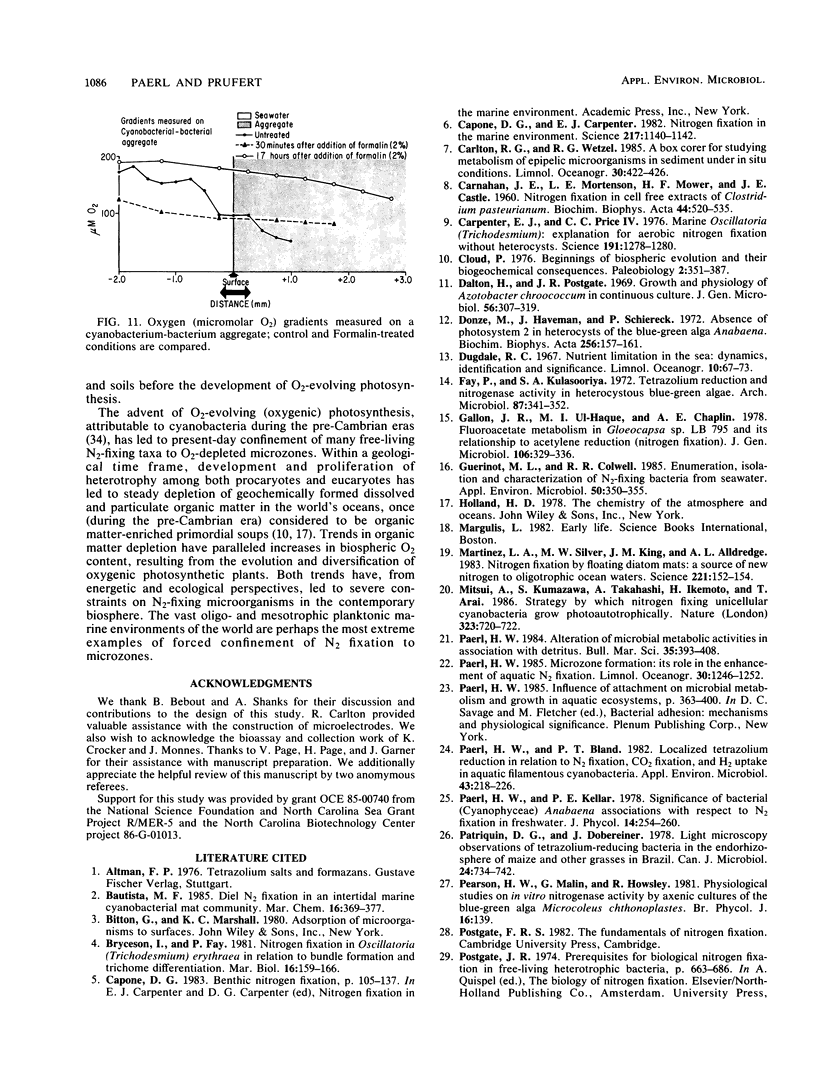
The nitrogen-deficient coastal waters of North Carolina contain suspended bacteria potentially able to fix N2. Bioassays aimed at identifying environmental factors controlling the development and proliferation of N2 fixation showed that dissolved organic carbon (as simple sugars and sugar alcohols) and particulate organic carbon (derived from Spartina alterniflora) additions elicited and enhanced N2 fixation (nitrogenase activity) in these waters. Nitrogenase activity occurred in samples containing flocculent, mucilage-covered bacterial aggregates. Cyanobacterium-bacterium aggregates also revealed N2 fixation. In all cases bacterial N2 fixation occurred in association with surficial microenvironments or microzones. Since nitrogenase is oxygen labile, we hypothesized that the aggregates themselves protected their constituent microbes from O2. Microelectrode O2 profiles revealed that aggregates had lower internal O2 tensions than surrounding waters. Tetrazolium salt (2,3,5-triphenyl-3-tetrazolium chloride) reduction revealed that patchy zones existed both within microbes and extracellularly in the mucilage surrounding microbes where free O2 was excluded. Triphenyltetrazolium chloride reduction also strongly inhibited nitrogenase activity. These findings suggest that N2 fixation is mediated by the availability of the appropriate types of reduced microzones. Organic carbon enrichment appears to serve as an energy and structural source for aggregate formation, both of which were required for eliciting N2 fixation responses of these waters.
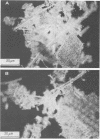
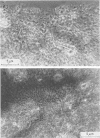
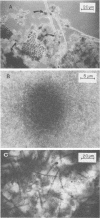
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARNAHAN J. E., MORTENSON L. E., MOWER H. F., CASTLE J. E. Nitrogen fixation in cell-free extracts of Clostridium pasteurianum. Biochim Biophys Acta. 1960 Nov 18;44:520–535. doi: 10.1016/0006-3002(60)91606-1. [DOI] [PubMed] [Google Scholar]
- Capone D. G., Carpenter E. J. Nitrogen fixation in the marine environment. Science. 1982 Sep 17;217(4565):1140–1142. doi: 10.1126/science.217.4565.1140. [DOI] [PubMed] [Google Scholar]
- Carpenter E. J., Price C. C. Marine oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976 Mar 26;191(4233):1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Growth and physiology of Azotobacter chroococcum in continuous culture. J Gen Microbiol. 1969 Jun;56(3):307–319. doi: 10.1099/00221287-56-3-307. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Guerinot M. L., Colwell R. R. Enumeration, isolation, and characterization of n(2)-fixing bacteria from seawater. Appl Environ Microbiol. 1985 Aug;50(2):350–355. doi: 10.1128/aem.50.2.350-355.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez L., Silver M. W., King J. M., Alldredge A. L. Nitrogen fixation by floating diatom mats: a source of new nitrogen to oligotrophic ocean waters. Science. 1983 Jul 8;221(4606):152–154. doi: 10.1126/science.221.4606.152. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Bland P. T. Localized Tetrazolium Reduction in Relation to N(2) Fixation, CO(2) Fixation, and H(2) Uptake in Aquatic Filamentous Cyanobacteria. Appl Environ Microbiol. 1982 Jan;43(1):218–226. doi: 10.1128/aem.43.1.218-226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Oxygen Microelectrode That Is Insensitive to Medium Chemical Composition: Use in an Acid Microbial Mat Dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983 Mar;45(3):755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Haystead A., Pearson H. W. Nitrogenase activity in heterocysts of blue-green algae. Nature. 1969 Oct 18;224(5216):226–228. doi: 10.1038/224226a0. [DOI] [PubMed] [Google Scholar]
- Tjepkema J., Van Berkum P. Acetylene reduction by soil cores of maize and sorghum in Brazil. Appl Environ Microbiol. 1977 Mar;33(3):626–629. doi: 10.1128/aem.33.3.626-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I., Barraquio W. L., De Guzman M. R., Cabrera D. A. Nitrogen-fixing (acetylene redution) activity and population of aerobic heterotrophic nitrogen-fixing bacteria associated with wetland rice. Appl Environ Microbiol. 1979 May;37(5):813–819. doi: 10.1128/aem.37.5.813-819.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]