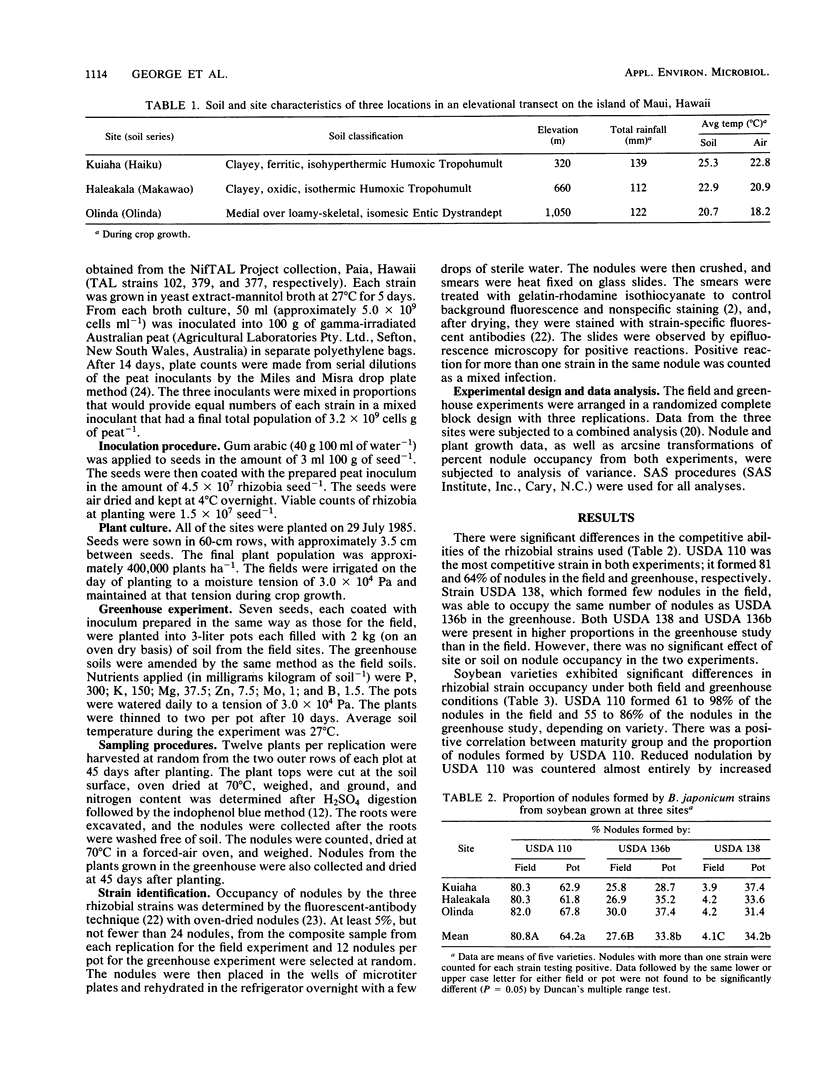
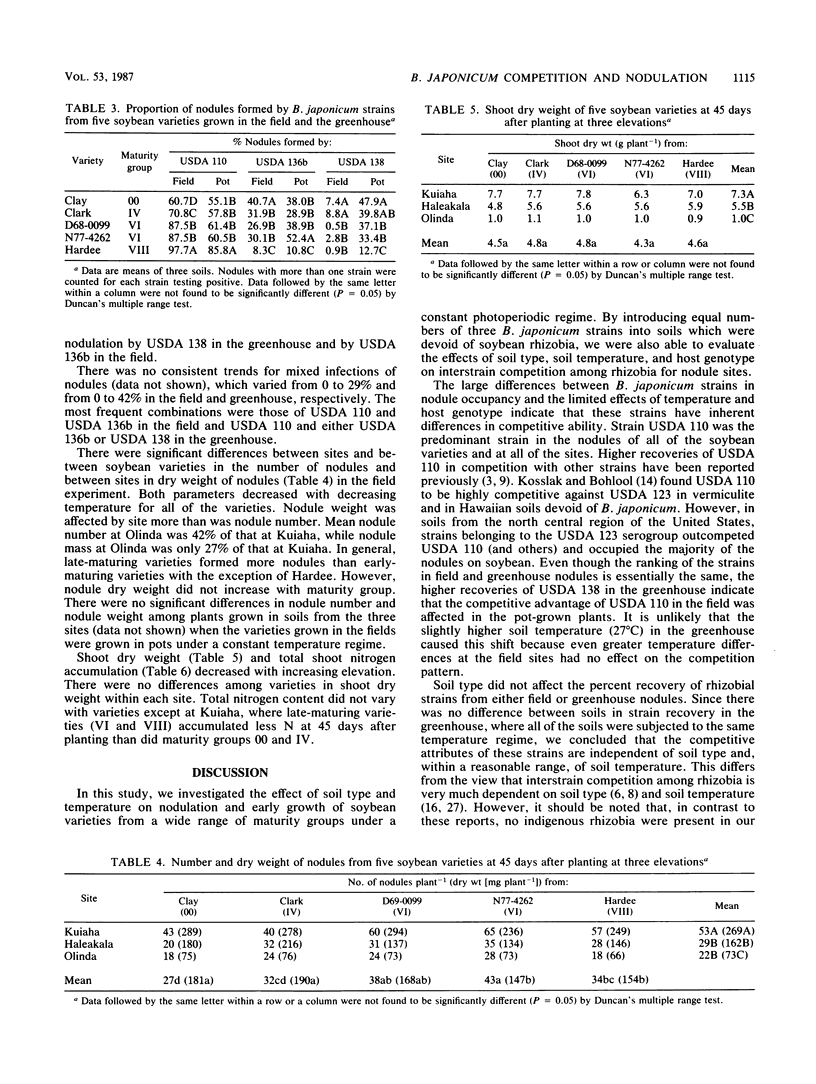
Abstract
The effects of temperature and soil type on interstrain competition of Bradyrhizobium japonicum and on nodulation and nitrogen accumulation in five soybean varieties belonging to four maturity groups were investigated at three sites devoid of soybean rhizobia along an elevational transect in Hawaii. Competition patterns of the three B. japonicum strains were unaffected by soil type or soil temperature. Strain USDA 110 was the best competitor, occupying on the average 81 and 64% of the nodules in the field and greenhouse experiments, respectively. Strain USDA 138 was the least successful in the field (4%), although it formed 34% of the nodules in the greenhouse. Nodule occupancy by B. japonicum strains was found to be related to soybean maturity group. Strain USDA 110 formed 61, 71, 88, 88, and 98% of the nodules in the field on Clay (00), Clark (IV), D68-0099 (VI), N77-4262 (VI), and Hardee (VIII), respectively. Strain USDA 136b formed few nodules on Hardee, an Rj2 soybean variety incompatible with that strain, in both experiments. Nodule number and weight at the 1,050-m site were reduced to 41 and 27%, respectively, of those at the 320-m site because of the decrease in temperature. Nodule number increased with increasing maturity group number at each site; however, there was not a corresponding increase in nodule weight. Nitrogen accumulation decreased from 246 mg of N per plant at the lowest elevation site to 26 mg of N per plant at the highest elevation. While soil type and temperature had no effect on strain competition, temperature had a profound influence on nodule parameters and plant growth.
Full text
PDF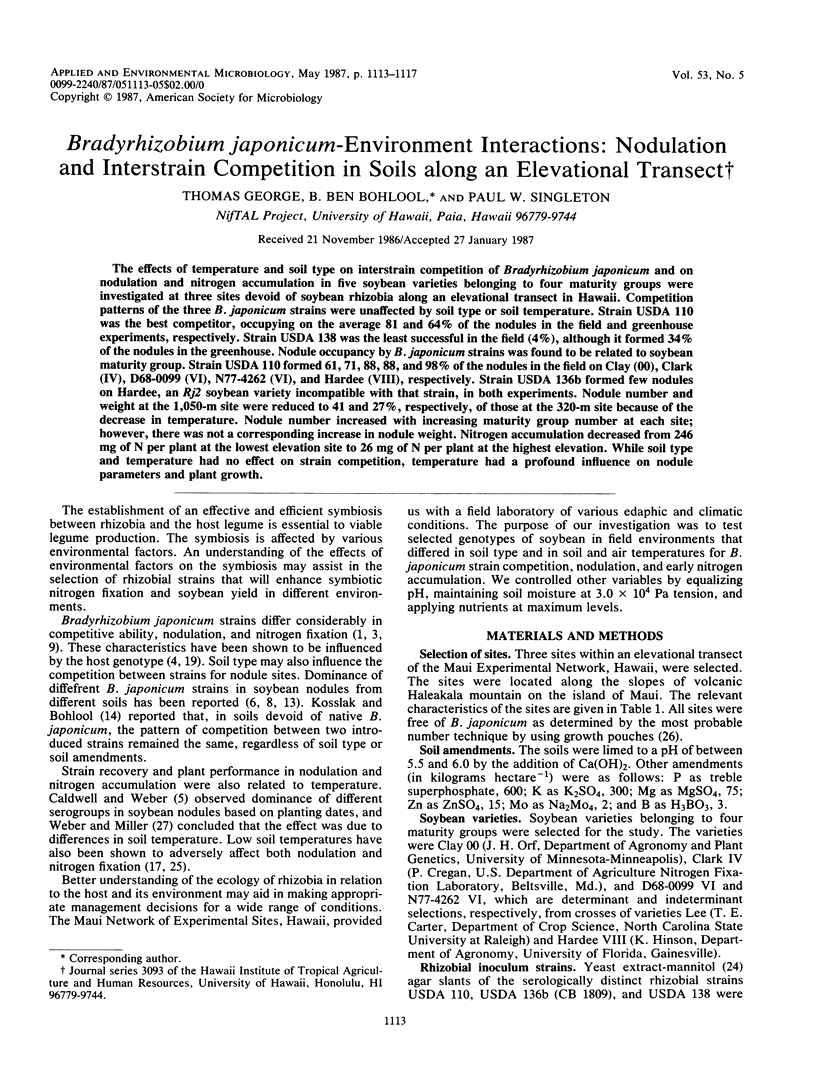


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Weber D. F., Uratsu S. L. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 1984 Apr;47(4):613–615. doi: 10.1128/aem.47.4.613-615.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Influence of Environmental Factors on Interstrain Competition in Rhizobium japonicum. Appl Environ Microbiol. 1985 May;49(5):1128–1133. doi: 10.1128/aem.49.5.1128-1133.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


