Abstract
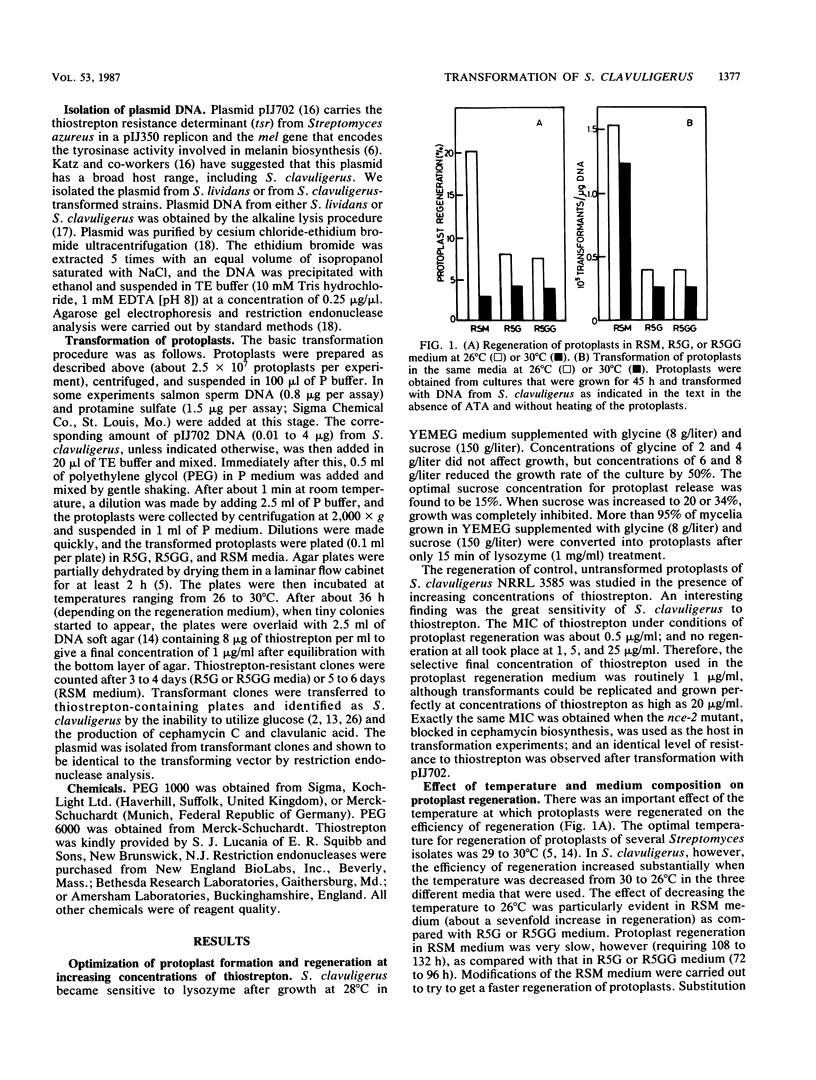
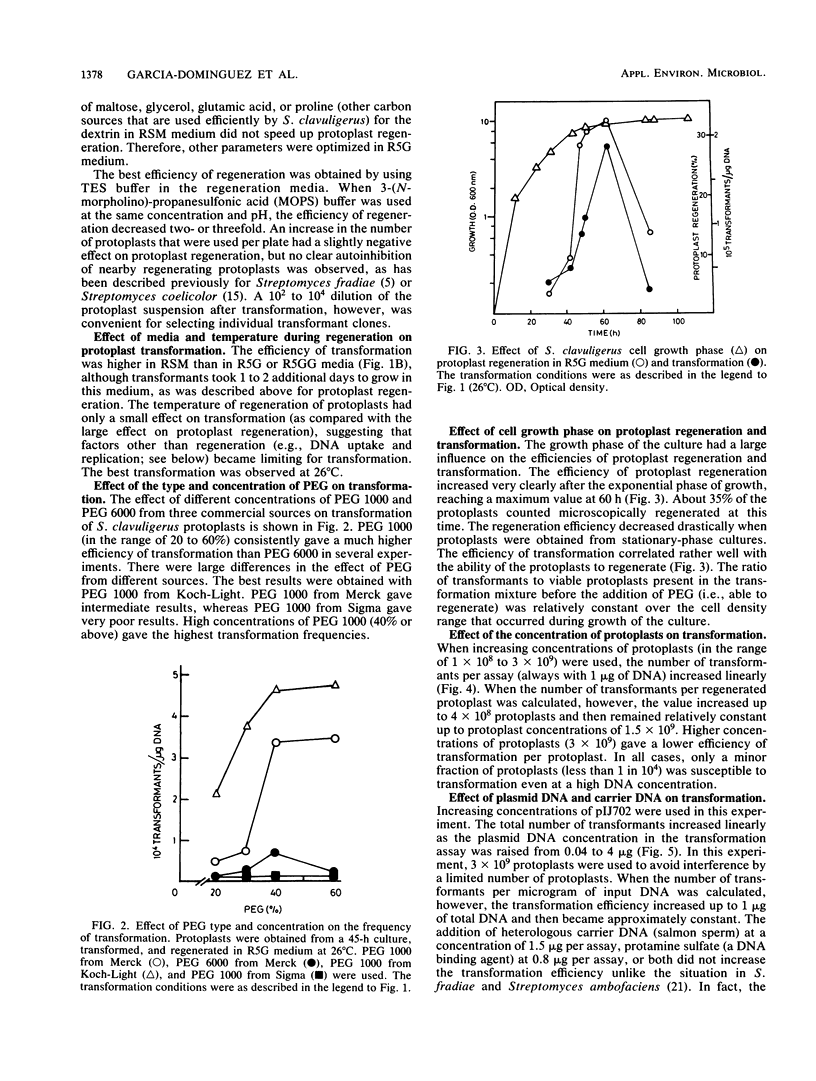
The conditions for optimal formation and regeneration of protoplasts of Streptomyces clavuligerus were established. The optimal temperature for regeneration of protoplasts and for transformation was 26 degrees C in three different regeneration media. The best efficiency of transformation was obtained with 40% polyethylene glycol 1000. The efficiencies of regeneration and transformation increased greatly when protoplasts were obtained from cultures in the early stationary phase of growth. The number of transformants per assay increased linearly with rising concentrations of protoplasts. However, the number of transformants per protoplast decreased at concentrations of protoplasts above 1.5 X 10(9). The total number of transformants rose linearly at increasing plasmid DNA concentrations, but the number of the transformants per microgram of DNA became constant at concentrations above 1 microgram of DNA. Transformation frequencies as high as 5 X 10(5) transformants per microgram of DNA were obtained when plasmid pIJ702 was isolated from S. clavuligerus but not when isolated from Streptomyces lividans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Demain A. L. Carbon catabolite regulation of cephalosporin production in Streptomyces clavuligerus. Antimicrob Agents Chemother. 1978 Aug;14(2):159–164. doi: 10.1128/aac.14.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Matsushima P. Protoplast fusion in Streptomyces: conditions for efficient genetic recombination and cell regeneration. J Gen Microbiol. 1981 Nov;127(1):137–146. doi: 10.1099/00221287-127-1-137. [DOI] [PubMed] [Google Scholar]
- Bernan V., Filpula D., Herber W., Bibb M., Katz E. The nucleotide sequence of the tyrosinase gene from Streptomyces antibioticus and characterization of the gene product. Gene. 1985;37(1-3):101–110. doi: 10.1016/0378-1119(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Cortés J., Liras P., Castro J. M., Martín J. F. Glucose regulation of cephamycin biosynthesis in Streptomyces lactamdurans is exerted on the formation of alpha-aminoadipyl-cysteinyl-valine and deacetoxycephalosporin C synthase. J Gen Microbiol. 1986 Jul;132(7):1805–1814. doi: 10.1099/00221287-132-7-1805. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M., Bibb M. J., Cohen S. N. Genetic recombination through protoplast fusion in Streptomyces. Nature. 1977 Jul 14;268(5616):171–174. doi: 10.1038/268171a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Matsushima P., Baltz R. H. Efficient plasmid transformation of Streptomyces ambofaciens and Streptomyces fradiae protoplasts. J Bacteriol. 1985 Jul;163(1):180–185. doi: 10.1128/jb.163.1.180-185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidcock K. A., Montenecourt B. S., Sands J. A. Genetic Recombination and Transformation in Protoplasts of Thermomonospora fusca. Appl Environ Microbiol. 1985 Sep;50(3):693–695. doi: 10.1128/aem.50.3.693-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodicio M. R., Chater K. F. Small DNA-free liposomes stimulate transfection of streptomyces protoplasts. J Bacteriol. 1982 Sep;151(3):1078–1085. doi: 10.1128/jb.151.3.1078-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J., Liras P., Martín J. F. Utilization of ornithine and arginine as specific precursors of clavulanic acid. Appl Environ Microbiol. 1986 Oct;52(4):892–897. doi: 10.1128/aem.52.4.892-897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Santamaria R. I., Gil J. A., Martin J. F. High-frequency transformation of Brevibacterium lactofermentum protoplasts by plasmid DNA. J Bacteriol. 1985 Apr;162(1):463–467. doi: 10.1128/jb.162.1.463-467.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Maurer K. H., Hutchinson C. R. Transformation of Streptomyces erythraeus. J Antibiot (Tokyo) 1986 Sep;39(9):1304–1313. doi: 10.7164/antibiotics.39.1304. [DOI] [PubMed] [Google Scholar]


