Abstract
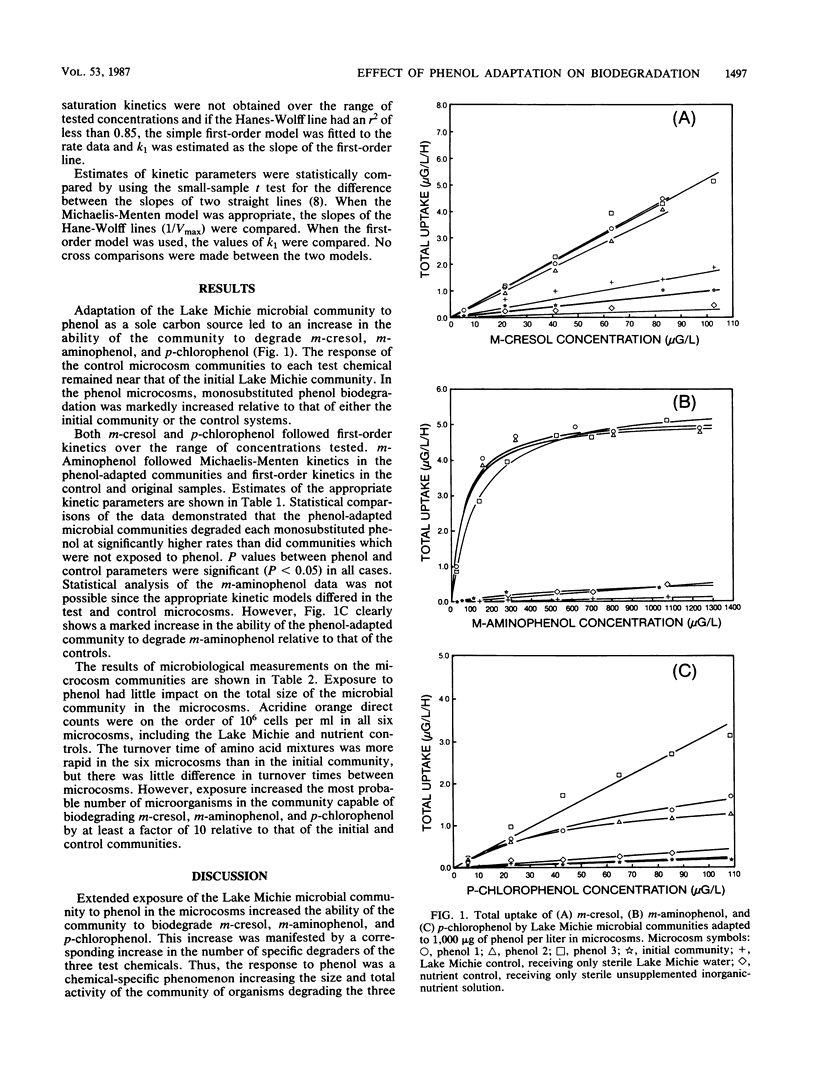
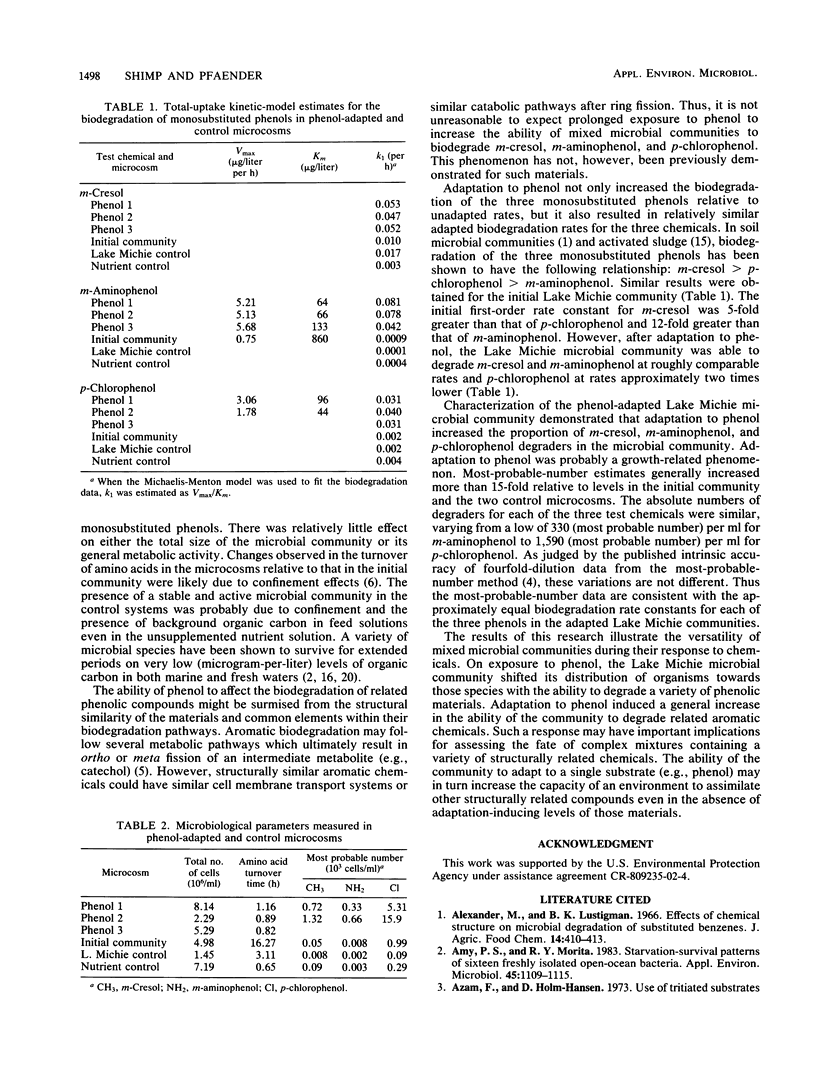
The adaptation of a mixed aquatic microbial community to phenol was examined in microcosms receiving phenol as a sole carbon source. Extended exposure (adaptation) to phenol resulted in adaptation of the microbial community to the structurally related aromatic compounds m-cresol, m-aminophenol, and p-chlorophenol. The increased biodegradation potential of the phenol-adapted microbial community was accompanied by a concurrent increase in the number of microorganisms able to degrade the three test compounds. Thus, adaptation to the three test chemicals was likely a growth-related result of extended exposure to phenol. The results indicate that adaptation to a single chemical may increase the assimilative capacity of an aquatic environment for other related chemicals even in the absence of adaptation-inducing levels of those materials.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPat-Polasko L. T., McCarty P. L., Zehnder A. J. Secondary substrate utilization of methylene chloride by an isolated strain of Pseudomonas sp. Appl Environ Microbiol. 1984 Apr;47(4):825–830. doi: 10.1128/aem.47.4.825-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmicke L. G., Williams R. T., Crawford R. L. 14C-most-probable-number method for enumeration of active heterotrophic microorganisms in natural waters. Appl Environ Microbiol. 1979 Oct;38(4):644–649. doi: 10.1128/aem.38.4.644-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Kollig H. P., Hodson R. E. Nutrient limitation and adaptation of microbial populations to chemical transformations. Appl Environ Microbiol. 1986 Mar;51(3):598–603. doi: 10.1128/aem.51.3.598-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Bartholomew G. W. Measurement of aquatic biodegradation rates by determining heterotrophic uptake of radiolabeled pollutants. Appl Environ Microbiol. 1982 Jul;44(1):159–164. doi: 10.1128/aem.44.1.159-164.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R. J., Pfaender F. K. Influence of easily degradable naturally occurring carbon substrates on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):394–401. doi: 10.1128/aem.49.2.394-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R., Pfaender F. K. Influence of naturally occurring humic acids on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):402–407. doi: 10.1128/aem.49.2.402-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. L., Alexander M. Role of resistance to starvation in bacterial survival in sewage and lake water. Appl Environ Microbiol. 1984 Aug;48(2):410–415. doi: 10.1128/aem.48.2.410-415.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A. Adaptation of natural microbial communities to degradation of xenobiotic compounds: effects of concentration, exposure time, inoculum, and chemical structure. Appl Environ Microbiol. 1983 Feb;45(2):428–435. doi: 10.1128/aem.45.2.428-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventullo R. M., Larson R. J. Adaptation of aquatic microbial communities to quaternary ammonium compounds. Appl Environ Microbiol. 1986 Feb;51(2):356–361. doi: 10.1128/aem.51.2.356-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


