Abstract
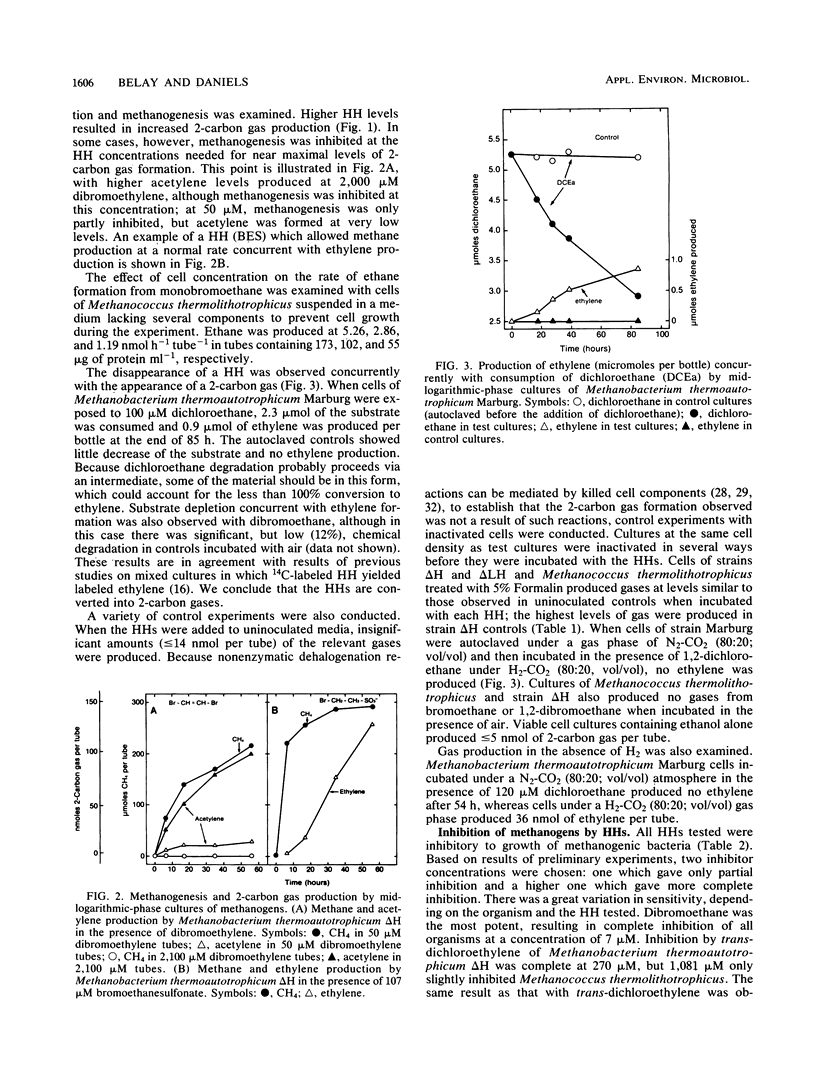
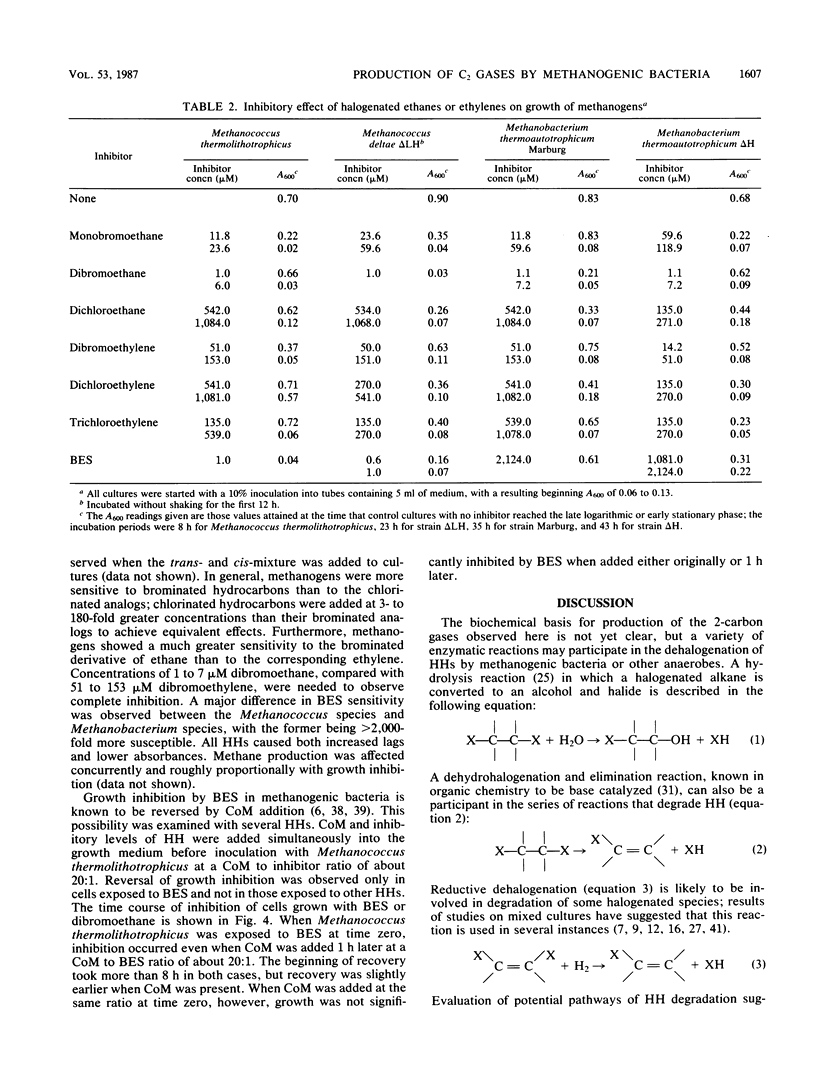
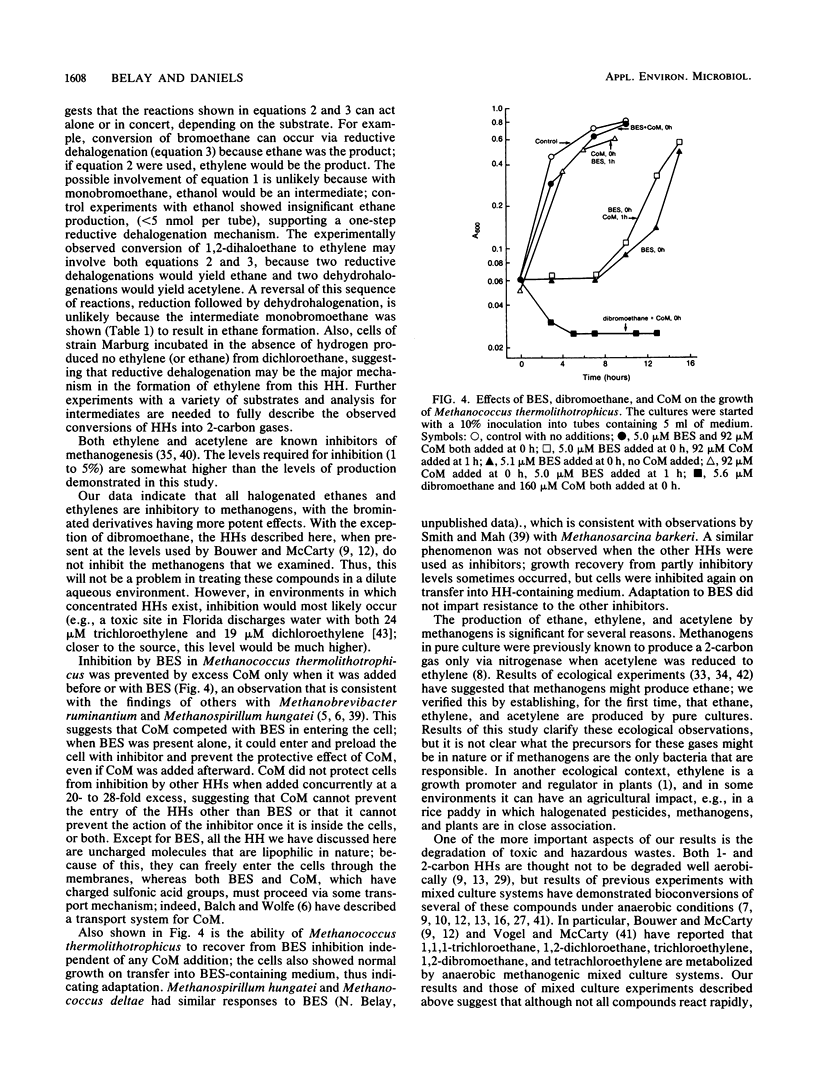
Several methanogenic bacteria were shown to produce ethane, ethylene, and acetylene when exposed to the halogenated hydrocarbons bromoethane, dibromo- or dichloroethane, and 1,2-dibromoethylene, respectively. They also produced ethylene when exposed to the coenzyme M analog and specific methanogenic inhibitor bromoethanesulfonic acid. The production of these gases from halogenated hydrocarbons has a variety of implications concerning microbial ecology, agriculture, and toxic waste treatment. All halogenated aliphatic compounds tested were inhibitory to methanogens. Methanococcus thermolithotrophicus, Methanococcus deltae, and Methanobacterium thermoautotrophicum ΔH and Marburg were completely inhibited by 7 μM 1,2-dibromoethane and, to various degrees, by 51 to 1,084 μM 1,2-dichloroethane, 1,2-dibromoethylene, 1,2-dichloroethylene, and trichloroethylene. In general, the brominated compounds were more inhibitory. The two Methanococcus species were fully inhibited by 1 μM bromoethanesulfonic acid, whereas both Methanobacterium strains were only partly inhibited by 2,124 μM. Coenzyme M protected cells from bromoethanesulfonic acid but not from any of the other inhibitors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson P. H. Chemicals from waste dumps. Science. 1985 Jul 26;229(4711):335–335. doi: 10.1126/science.229.4711.335. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Transport of coenzyme M (2-mercaptoethanesulfonic acid) in Methanobacterium ruminantium. J Bacteriol. 1979 Jan;137(1):264–273. doi: 10.1128/jb.137.1.264-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay N., Sparling R., Daniels L. Dinitrogen fixation by a thermophilic methanogenic bacterium. Nature. 1984 Nov 15;312(5991):286–288. doi: 10.1038/312286a0. [DOI] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Effects of 2-bromoethanesulfonic Acid and 2- chloroethanesulfonic Acid on acetate utilization in a continuous-flow methanogenic fixed-film column. Appl Environ Microbiol. 1983 Apr;45(4):1408–1410. doi: 10.1128/aem.45.4.1408-1410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Ethylene dibromide transformation under methanogenic conditions. Appl Environ Microbiol. 1985 Aug;50(2):527–528. doi: 10.1128/aem.50.2.527-528.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of halogenated organic compounds under denitrification conditions. Appl Environ Microbiol. 1983 Apr;45(4):1295–1299. doi: 10.1128/aem.45.4.1295-1299.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Daniels L., Belay N., Rajagopal B. S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol. 1986 Apr;51(4):703–709. doi: 10.1128/aem.51.4.703-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Giger W., Molnar-Kubica E. Tetrachloroethylene in contaminated ground and drinking waters. Bull Environ Contam Toxicol. 1978 Apr;19(4):475–480. doi: 10.1007/BF01685829. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Scheper A., Dijkhuizen L., Witholt B. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol. 1985 Mar;49(3):673–677. doi: 10.1128/aem.49.3.673-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisinger T. Microorganisms and xenobiotic compounds. Experientia. 1983 Nov 15;39(11):1183–1191. doi: 10.1007/BF01990355. [DOI] [PubMed] [Google Scholar]
- Murthy N. B., Kaufman D. D., Fries G. F. Degradation of pentachlorophenol (PCP) in aerobic and anaerobic soil. J Environ Sci Health B. 1979;14(1):1–14. doi: 10.1080/03601237909372110. [DOI] [PubMed] [Google Scholar]
- Oremland R. S. Microbial formation of ethane in anoxic estuarine sediments. Appl Environ Microbiol. 1981 Jul;42(1):122–129. doi: 10.1128/aem.42.1.122-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Taylor B. F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975 Oct;30(4):707–709. doi: 10.1128/am.30.4.707-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R. Reversal of 2-bromoethanesulfonate inhibition of methanogenesis in Methanosarcina sp. J Bacteriol. 1983 Nov;156(2):516–523. doi: 10.1128/jb.156.2.516-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Lenahan R., Kanik M. Impact of trichloroethylene contaminated groundwater discharged to the main canal and Indian River lagoon, Vero Beach, Florida. Bull Environ Contam Toxicol. 1985 Apr;34(4):578–586. doi: 10.1007/BF01609779. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Anguish T., Cardwell S. C. Selective inhibition by 2-bromoethanesulfonate of methanogenesis from acetate in a thermophilic anaerobic digestor. Appl Environ Microbiol. 1984 Jun;47(6):1343–1345. doi: 10.1128/aem.47.6.1343-1345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


