Abstract
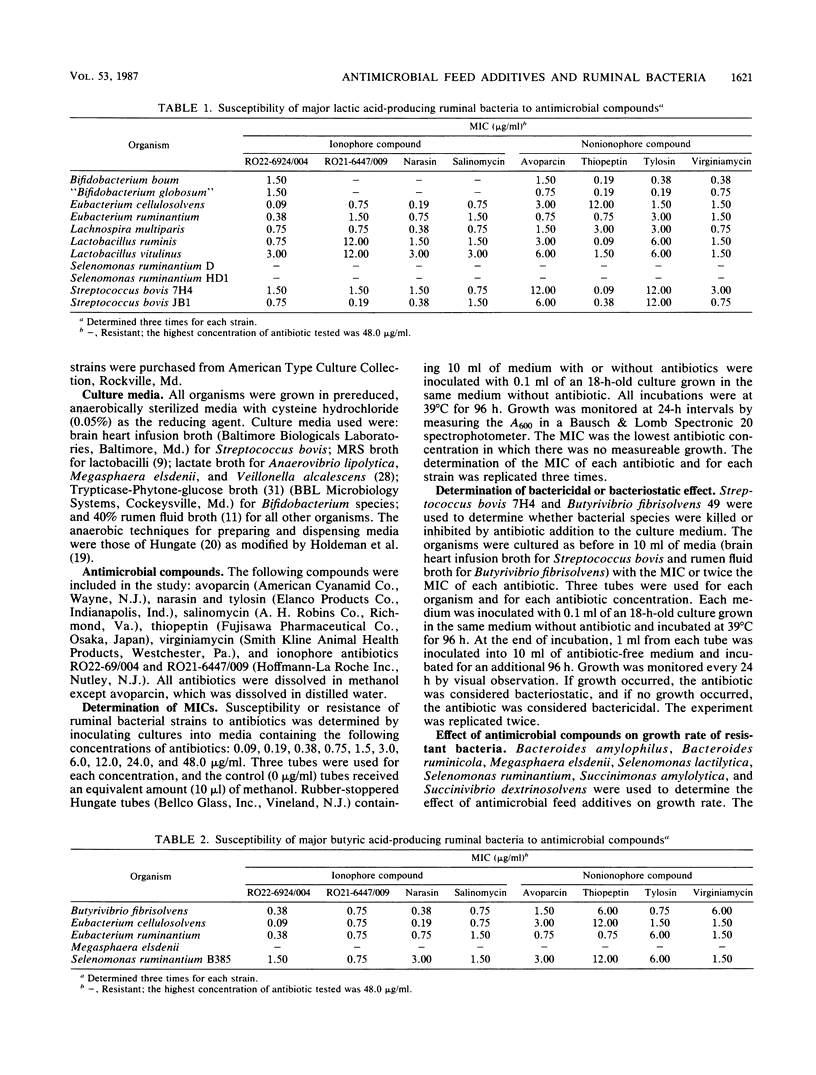
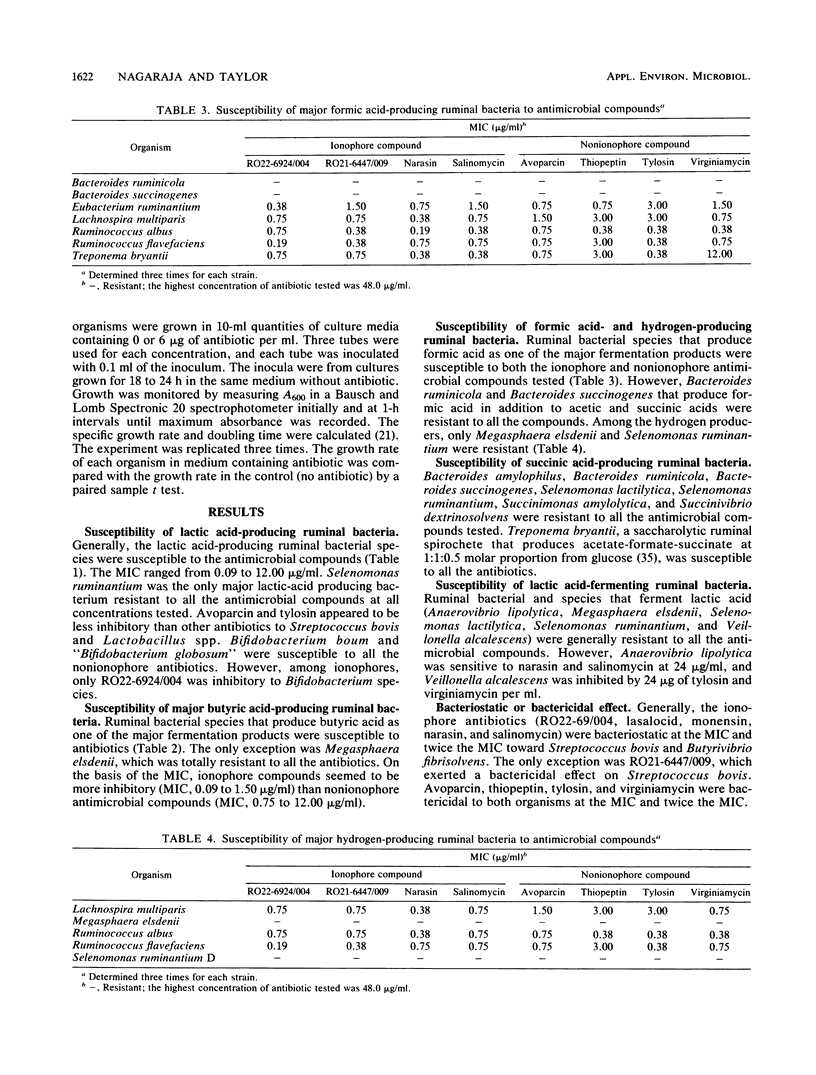
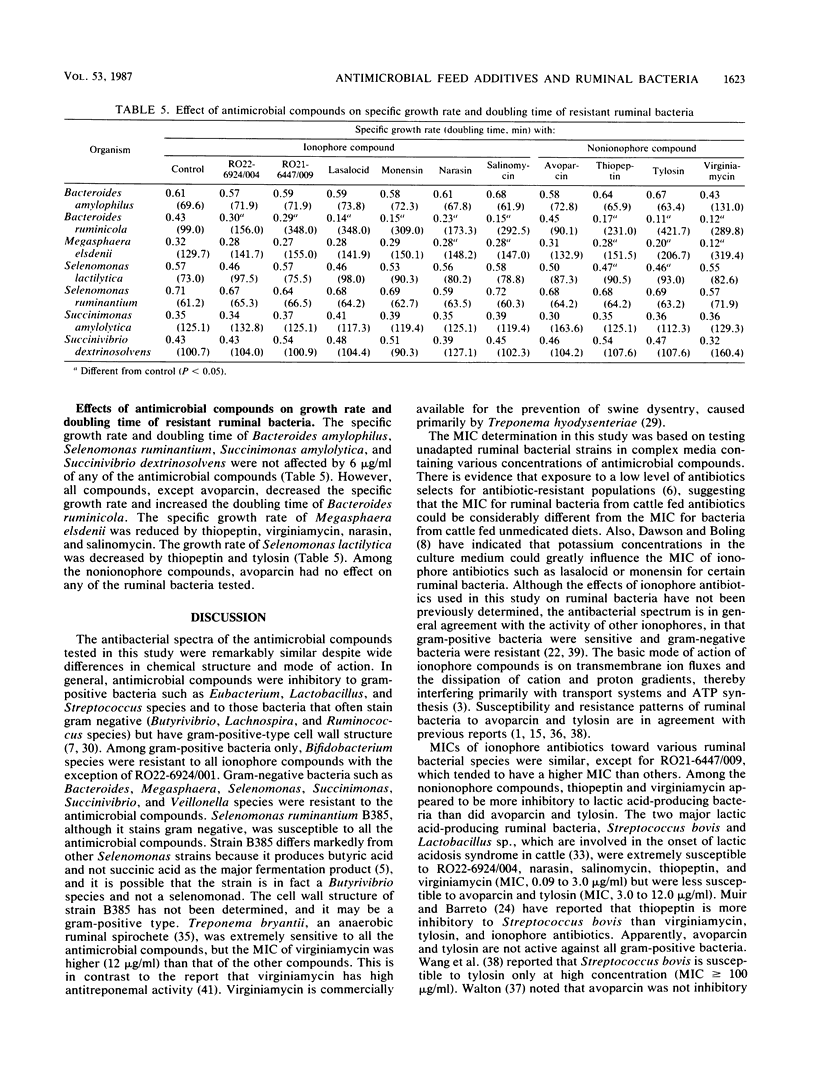
Susceptibility and resistance of ruminal bacterial species to avoparcin, narasin, salinomycin, thiopeptin, tylosin, virginiamycin, and two new ionophore antibiotics, RO22-6924/004 and RO21-6447/009, were determined. Generally, antimicrobial compounds were inhibitory to gram-positive bacteria and those bacteria that have gram-positive-like cell wall structure. MICs ranged from 0.09 to 24.0 micrograms/ml. Gram-negative bacteria were resistant at the highest concentration tested (48.0 micrograms/ml). On the basis of their fermentation products, ruminal bacteria that produce lactic acid, butyric acid, formic acid, or hydrogen were susceptible and bacteria that produce succinic acid or ferment lactic acid were resistant to the antimicrobial compounds. Selenomonas ruminantium was the only major lactic acid-producing bacteria resistant to all the antimicrobial compounds tested. Avoparcin and tylosin appeared to be less inhibitory (MIC greater than 6.0 micrograms/ml) than the other compounds to the two major lactic acid-producing bacteria, Streptococcus bovis and Lactobacillus sp. Ionophore compounds seemed to be more inhibitory (MIC, 0.09 to 1.50 micrograms/ml) than nonionophore compounds (MIC, 0.75 to 12.0 micrograms/ml) to the major butyric acid-producing bacteria. Treponema bryantii, an anaerobic rumen spirochete, was less sensitive to virginiamycin than to the other antimicrobial compounds. Ionophore compounds were generally bacteriostatic, and nonionophore compounds were bactericidal. The specific growth rate of Bacteroides ruminicola was reduced by all the antimicrobial compounds except avoparcin. The antibacterial spectra of the feed additives were remarkably similar, and it appears that MICs may not be good indicators of the potency of the compounds in altering ruminal fermentation characteristics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J Bacteriol. 1956 Aug;72(2):162–167. doi: 10.1128/jb.72.2.162-167.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen W. G., Bates D. B. Ionophores: their effect on production efficiency and mode of action. J Anim Sci. 1984 Jun;58(6):1465–1483. doi: 10.2527/jas1984.5861465x. [DOI] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of Butyrivibrio fibrisolvens: a gram-positive bacterium. J Bacteriol. 1977 Mar;129(3):1506–1512. doi: 10.1128/jb.129.3.1506-1512.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effects of lasalocid or monensin on lactate-producing or -using rumen bacteria. J Anim Sci. 1981 Feb;52(2):418–426. doi: 10.2527/jas1981.522418x. [DOI] [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Sensitivity and resistance to growth promoting agents in animal lactobacilli. J Appl Bacteriol. 1981 Oct;51(2):283–288. doi: 10.1111/j.1365-2672.1981.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Dyer I. A., Koes R. M., Herlugson M. L., Ojikutu L. B., Preston R. L., Zimmer P., DeLay R. Effect of avoparcin and monensin on performance of finishing heifers. J Anim Sci. 1980 Oct;51(4):843–846. doi: 10.2527/jas1980.514843x. [DOI] [PubMed] [Google Scholar]
- Froetschel M. A., Croom W. J., Jr, Gaskins H. R., Leonard E. S., Whitacre M. D. Effects of avoparcin on ruminal propionate production and amino acid degradation in sheep fed high and low fiber diets. J Nutr. 1983 Jul;113(7):1355–1362. doi: 10.1093/jn/113.7.1355. [DOI] [PubMed] [Google Scholar]
- Fulghum R. S., Baldwin B. B., Williams P. P. Antibiotic susceptibility of anaerobic ruminal bacteria. Appl Microbiol. 1968 Feb;16(2):301–307. doi: 10.1128/am.16.2.301-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich R. D., Garrett J. E., Gast D. R., Kirick M. A., Larson D. A., Meiske J. C. Influence of monensin on the performance of cattle. J Anim Sci. 1984 Jun;58(6):1484–1498. doi: 10.2527/jas1984.5861484x. [DOI] [PubMed] [Google Scholar]
- Merchen N. R., Berger L. L. Effect of salinomycin level on nutrient digestibility and ruminal characteristics of sheep and feedlot performance of cattle. J Anim Sci. 1985 May;60(5):1338–1346. doi: 10.2527/jas1985.6051338x. [DOI] [PubMed] [Google Scholar]
- Muir L. A., Barreto A., Jr Sensitivity of Streptococcus bovis to various antibiotics. J Anim Sci. 1979 Mar;48(3):468–473. doi: 10.2527/jas1979.483468x. [DOI] [PubMed] [Google Scholar]
- Muir L. A., Rickes E. L., Duquette P. F., Smith G. E. Control of wheat-induced lactic acidosis in sheep by thiopeptin and related antibiotics. J Anim Sci. 1980 Mar;50(3):547–553. doi: 10.2527/jas1980.503547x. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Avery T. B., Bartley E. E., Roof S. K., Dayton A. D. Effect of lasalocid, monensin or thiopeptin on lactic acidosis in cattle. J Anim Sci. 1982 Mar;54(3):649–658. doi: 10.2527/jas1982.543649x. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Avery T. B., Galitzer S. J., Harmon D. L. Effect of ionophore antibiotics on experimentally induced lactic acidosis in cattle. Am J Vet Res. 1985 Dec;46(12):2444–2452. [PubMed] [Google Scholar]
- Nagaraja T. G., Fina L. R., Lassman B. A., Bartley E. E., Anthony H. D., Sapienza D. A., Brent B. E. Characterization of endotoxin from the rumen bacterium Megasphaera elsdenii. Am J Vet Res. 1979 Jan;40(1):35–39. [PubMed] [Google Scholar]
- Olson L. D., Rodabaugh D. E. Evaluation of virginiamycin in feed for treatment and retreatment of swine dysentery. Am J Vet Res. 1977 Oct;38(10):1485–1490. [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardovi V., Trovatelli L. D., Crociani F., Sgorbati B. Bifid bacteria in bovine rumen. New species of the genus Bifidobacterium: B. globosum n.sp. and B. ruminale n.sp. Arch Mikrobiol. 1969;68(3):278–294. [PubMed] [Google Scholar]
- Schelling G. T. Monensin mode of action in the rumen. J Anim Sci. 1984 Jun;58(6):1518–1527. doi: 10.2527/jas1984.5861518x. [DOI] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Spires H. R., Algeo J. W. Laidlomycin butyrate--an ionophore with enhanced intraruminal activity. J Anim Sci. 1983 Dec;57(6):1553–1560. doi: 10.2527/jas1983.5761553x. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch Microbiol. 1980 Sep;127(2):145–156. doi: 10.1007/BF00428018. [DOI] [PubMed] [Google Scholar]
- Walton J. R. The effect of dietary avoparcin on the antibiotic resistance patterns of enteric and pharyngeal bacteria isolated from broiler chickens. Zentralbl Veterinarmed B. 1978 May;25(4):290–300. doi: 10.1111/j.1439-0450.1978.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Wang C. L., Baldwin B. B., Fulghum R. S., Williams P. P. Quantitative antibiotic sensitivities of ruminal bacteria. Appl Microbiol. 1969 Oct;18(4):677–679. doi: 10.1128/am.18.4.677-679.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Watanabe J., Kuramitsu S., Maruyama H. B. Comparison of the activity of ionophores with other antibacterial agents against anaerobes. Antimicrob Agents Chemother. 1981 Apr;19(4):519–525. doi: 10.1128/aac.19.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. J., Shively J. E. In vitro antitreponemal activities of carbadox, virginiamycin, olaquindox, and tylosin as indices of their effectiveness for preventing swine dysentery. Vet Med Small Anim Clin. 1978 Mar;73(3):349–351. [PubMed] [Google Scholar]
- el Akkad I., Hobson P. N. Effect of antibiotics on some rumen and intestinal bacteria. Nature. 1966 Mar 5;209(5027):1046–1047. doi: 10.1038/2091046a0. [DOI] [PubMed] [Google Scholar]


