Abstract
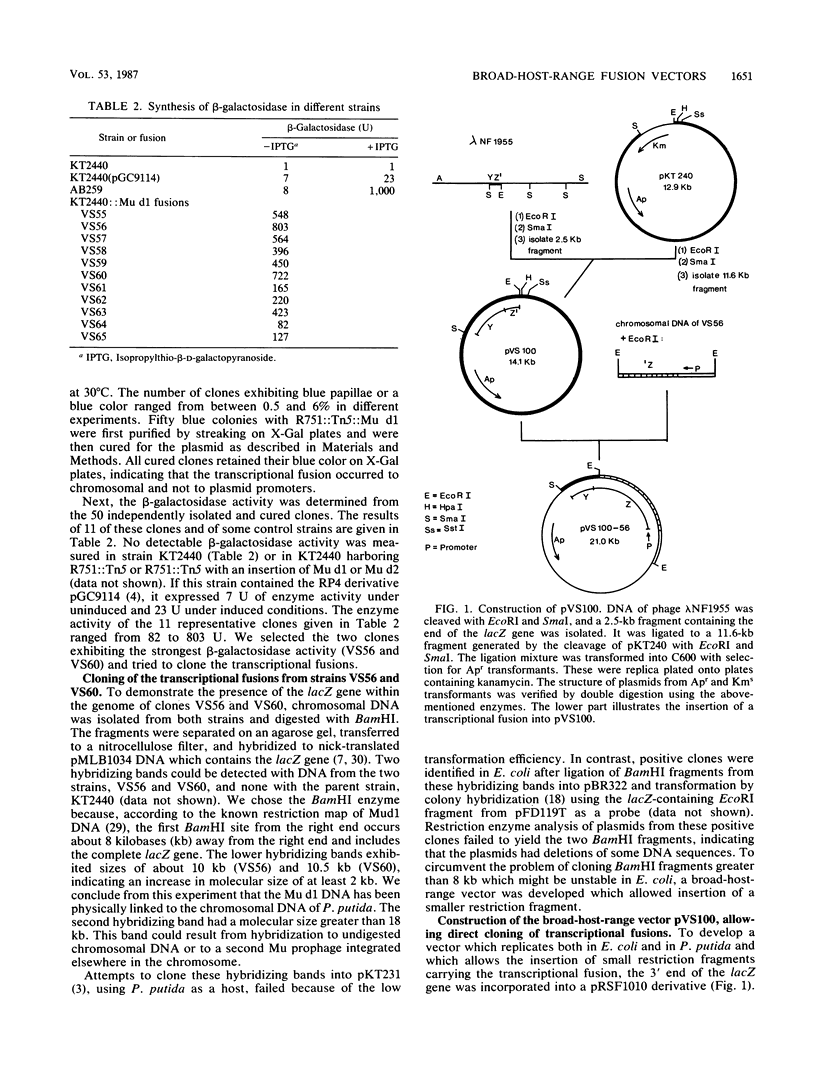
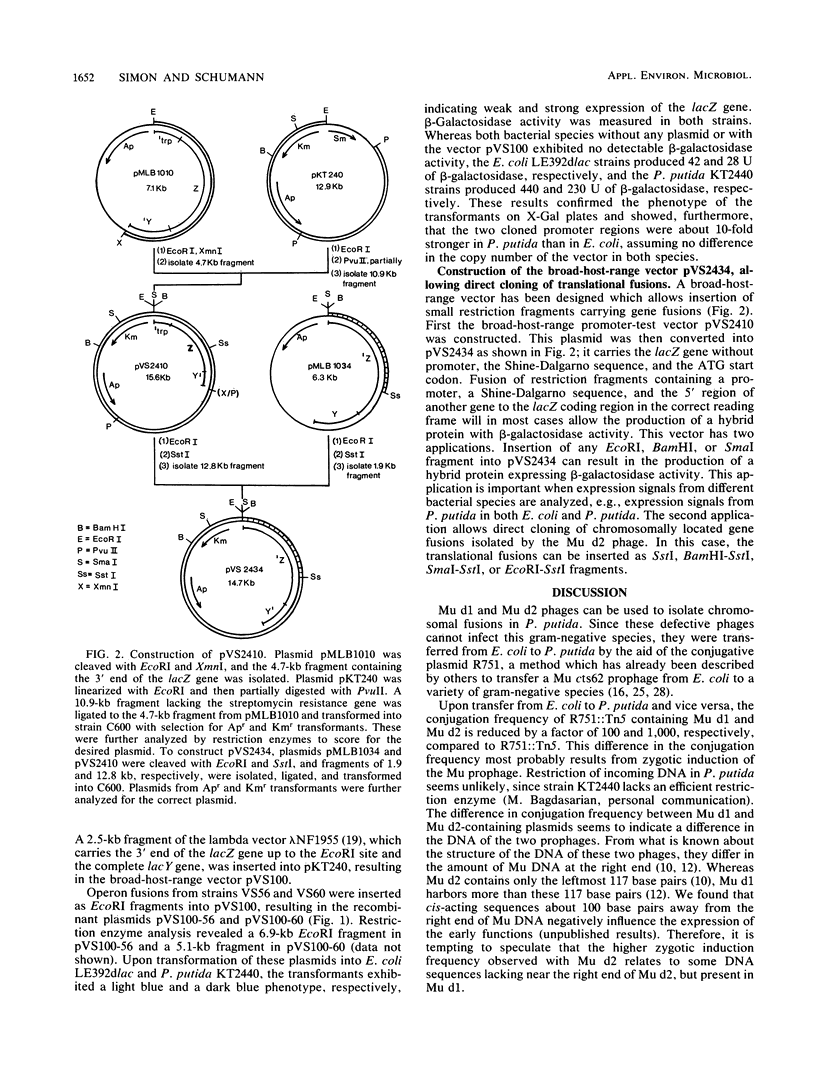
The Mu d1 and Mu d2 prophages were integrated into the conjugative broad-host-range plasmid R751. The two plasmids were then transferred into Pseudomonas putida, and derivatives carrying intact Mu prophages were recovered. After induction of Mu at 42 degrees C, both operon and gene fusions were observed on 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-Gal) plates. Broad-host-range vectors were constructed which allow direct cloning of both operon or gene fusions and their analysis in Escherichia coli and P. putida. By using one of these vectors, two operon fusions were isolated from the P. putida chromosome and comparatively analyzed in E. coli and P. putida.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Baumberg S., Cornelis G., Panagiotakopoulos M., Roberts M. Expression of the lactose transposon Tn951 in Escherichia coli, Proteus and Pseudomonas. J Gen Microbiol. 1980 Jul;119(1):257–262. doi: 10.1099/00221287-119-1-257. [DOI] [PubMed] [Google Scholar]
- Beny G., Boyen A., Charlier D., Lissens W., Feller A., Glansdorff N. Promoter mapping and selection of operator mutants by using insertion of bacteriophage Mu in the argECBH divergent operon of Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):62–67. doi: 10.1128/jb.151.1.62-67.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. Overproduction of the Tn3 transposition protein and its role in DNA transposition. Cell. 1982 Feb;28(2):345–354. doi: 10.1016/0092-8674(82)90352-x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Cordes K., Berman M. L., Das A. Mutagenesis and mutation transfer induced by ultraviolet light in plasmid-cloned DNA. Gene. 1984 Feb;27(2):213–222. doi: 10.1016/0378-1119(84)90142-2. [DOI] [PubMed] [Google Scholar]
- Chou J., Lemaux P. G., Casadaban M. J., Cohen S. N. Transposition protein of Tn3: identification and characterisation of an essential repressor-controlled gene product. Nature. 1979 Dec 20;282(5741):801–806. doi: 10.1038/282801a0. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Howe M. M., Ingraham J. L., Pierson L. S., 3rd, Turnbough C. L., Jr Infection of Salmonella typhimurium with coliphage Mu d1 (Apr lac): construction of pyr::lac gene fusions. J Bacteriol. 1981 Jan;145(1):299–305. doi: 10.1128/jb.145.1.299-305.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Eady R. R., Espin G., Hill S., Iaccarino M., Kahn D., Merrick M. Analysis of regulation of Klebsiella pneumoniae nitrogen fixation (nif) gene cluster with gene fusions. Nature. 1980 Jul 10;286(5769):128–132. doi: 10.1038/286128a0. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hui I., Maltman K., Little R., Hastrup S., Johnsen M., Fiil N., Dennis P. Insertions of transposon Tn5 into ribosomal protein PNA polymerase operons. J Bacteriol. 1982 Dec;152(3):1022–1032. doi: 10.1128/jb.152.3.1022-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Asai Y., Nakazawa A., Nakazawa T. Nucleotide sequence of a DNA segment promoting transcription in Pseudomonas putida. J Bacteriol. 1986 Jun;166(3):739–745. doi: 10.1128/jb.166.3.739-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka N., Nakamura S., Inuzuka M., Tomoeda M. Specific action of sodium dodecyl sulfate on the sex factor of Escherichia coli K-12 Hfr strains. J Bacteriol. 1969 Nov;100(2):827–835. doi: 10.1128/jb.100.2.827-835.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Sur les processus de conjugaison et de recombinaison chez Escherichia coli. I. L'induction par conjugaison ou induction zygotique. Ann Inst Pasteur (Paris) 1956 Oct;91(4):486–510. [PubMed] [Google Scholar]
- Jobanputra R. S., Datta N. Trimethoprim R factors in enterobacteria from clinical specimens. J Med Microbiol. 1974 May;7(2):169–177. doi: 10.1099/00222615-7-2-169. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Heffernan L., Wilcox G. Isolation of ara-lac gene fusions in Salmonella typhimurium LT2 by using transducing bacteriophage Mu d (Apr lac). J Bacteriol. 1980 Sep;143(3):1325–1331. doi: 10.1128/jb.143.3.1325-1331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Takizawa N., Harada T. Introduction of bacteriophage Mu into bacteria of various genera and intergeneric gene transfer by RP4::Mu. J Bacteriol. 1981 Jan;145(1):358–368. doi: 10.1128/jb.145.1.358-368.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M. B., Malamy M. H. A new insertion sequence, IS121, is found on the Mu dI1 (Ap lac) bacteriophage and the Escherichia coli K-12 chromosome. J Bacteriol. 1983 Nov;156(2):669–679. doi: 10.1128/jb.156.2.669-679.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schumann W., Bade E. G. In vitro constructed plasmids containing both ends of bacteriophage Mu DNA express phage functions. Mol Gen Genet. 1979 Jan 16;169(1):97–105. doi: 10.1007/BF00267550. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Sporn P. Tn402: a new transposable element determining trimethoprim resistance that inserts in bacteriophage lambda. J Bacteriol. 1977 Mar;129(3):1632–1635. doi: 10.1128/jb.129.3.1632-1635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


