Abstract
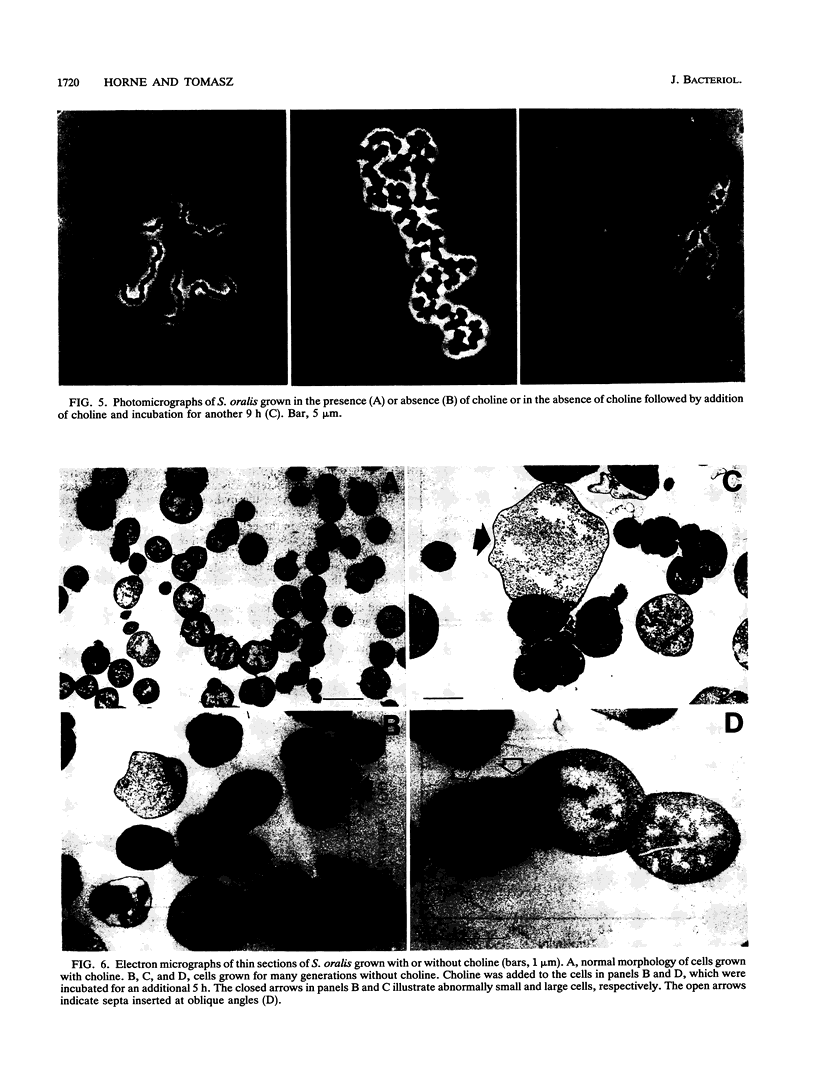
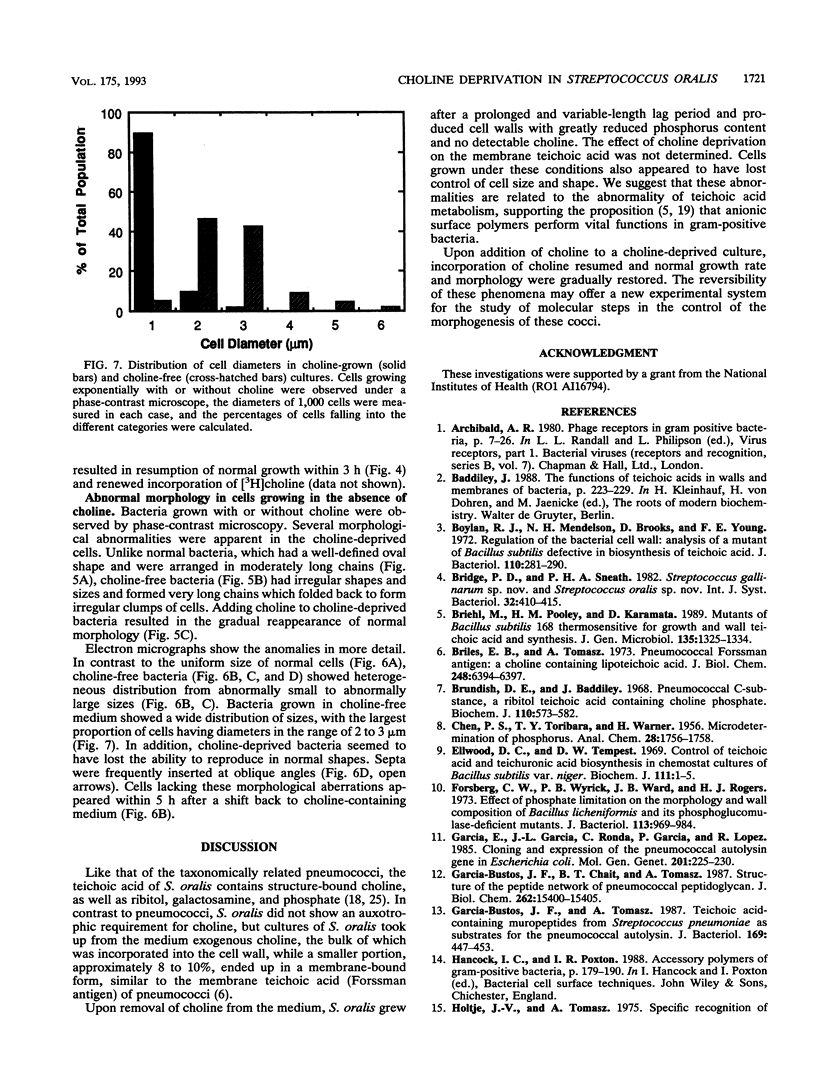
Streptococcus oralis ATCC 35037 took up radioactively labeled choline from growth medium. Most of the choline (80 to 90%) was incorporated into the cell wall teichoic acid, and about 10% was localized in the plasma membrane. While cells grew in choline-free medium, they did so at slow rates and produced cell walls with greatly reduced amounts of phosphate and no detectable choline. Cells grown in choline-free medium had grossly abnormal shape and size. Both biochemical and morphological abnormalities were reversible by addition of choline to the medium.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Chait B. T., Tomasz A. Structure of the peptide network of pneumococcal peptidoglycan. J Biol Chem. 1987 Nov 15;262(32):15400–15405. [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Tomasz A. Teichoic acid-containing muropeptides from Streptococcus pneumoniae as substrates for the pneumococcal autolysin. J Bacteriol. 1987 Feb;169(2):447–453. doi: 10.1128/jb.169.2.447-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., García J. L., Ronda C., García P., López R. Cloning and expression of the pneumococcal autolysin gene in Escherichia coli. Mol Gen Genet. 1985;201(2):225–230. doi: 10.1007/BF00425663. [DOI] [PubMed] [Google Scholar]
- Honeyman A. L., Stewart G. C. Identification of the protein encoded by rodC, a cell division gene from Bacillus subtilis. Mol Microbiol. 1988 Nov;2(6):735–741. doi: 10.1111/j.1365-2958.1988.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Pneumococcal Forssman antigen: enrichment in mesosomal membranes and specific binding to the autolytic enzyme of Streptococcus pneumoniae. J Bacteriol. 1985 Jan;161(1):18–24. doi: 10.1128/jb.161.1.18-24.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Young N. M. Structure of the complex polysaccharide C-substance from Streptococcus pneumoniae type 1. Biochemistry. 1980 Sep 30;19(20):4712–4719. doi: 10.1021/bi00561a026. [DOI] [PubMed] [Google Scholar]
- Karamata D., Pooley H. M., Monod M. Expression of heterologous genes for wall teichoic acid in Bacillus subtilis 168. Mol Gen Genet. 1987 Apr;207(1):73–81. doi: 10.1007/BF00331493. [DOI] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H. Bacterial growth and division: genes, structures, forces, and clocks. Microbiol Rev. 1982 Sep;46(3):341–375. doi: 10.1128/mr.46.3.341-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Schmidhuber S., Ludwig W., Schleifer K. H. Construction of a DNA probe for the specific identification of Streptococcus oralis. J Clin Microbiol. 1988 May;26(5):1042–1044. doi: 10.1128/jcm.26.5.1042-1044.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967 Aug 11;157(3789):694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Westphal M., Briles E. B., Fletcher P. On the physiological functions of teichoic acids. J Supramol Struct. 1975;3(1):1–16. doi: 10.1002/jss.400030102. [DOI] [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]