Abstract
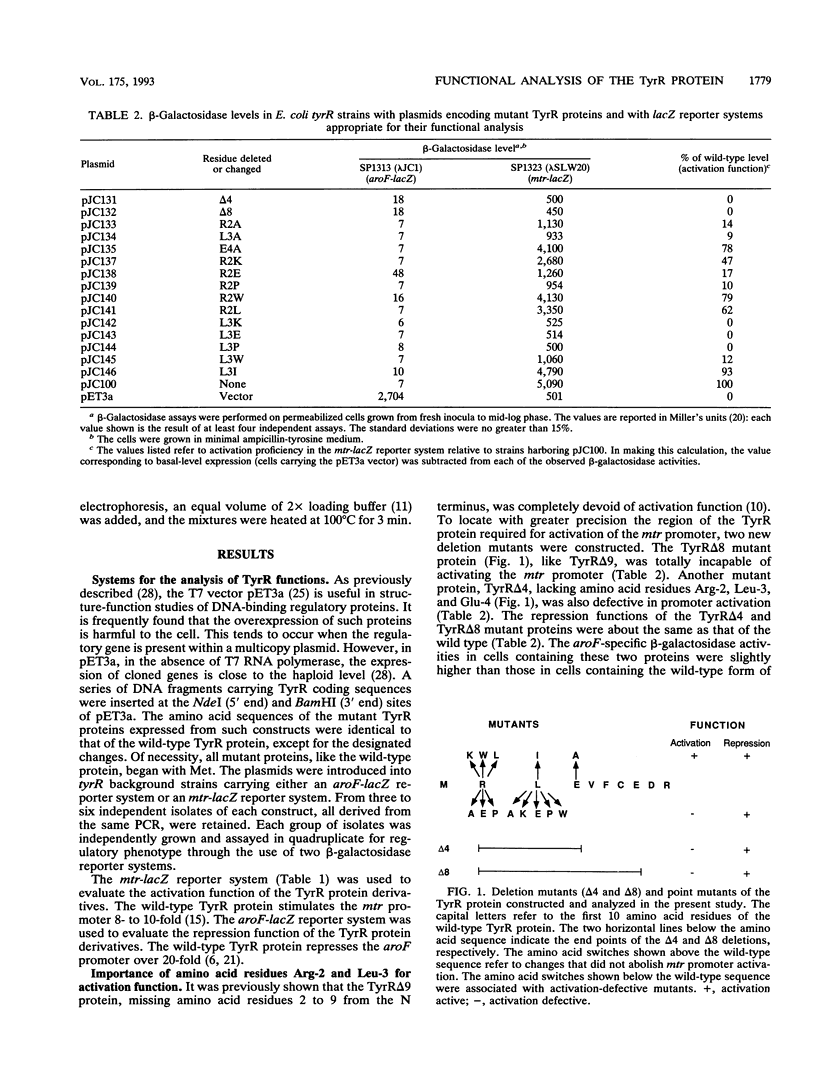
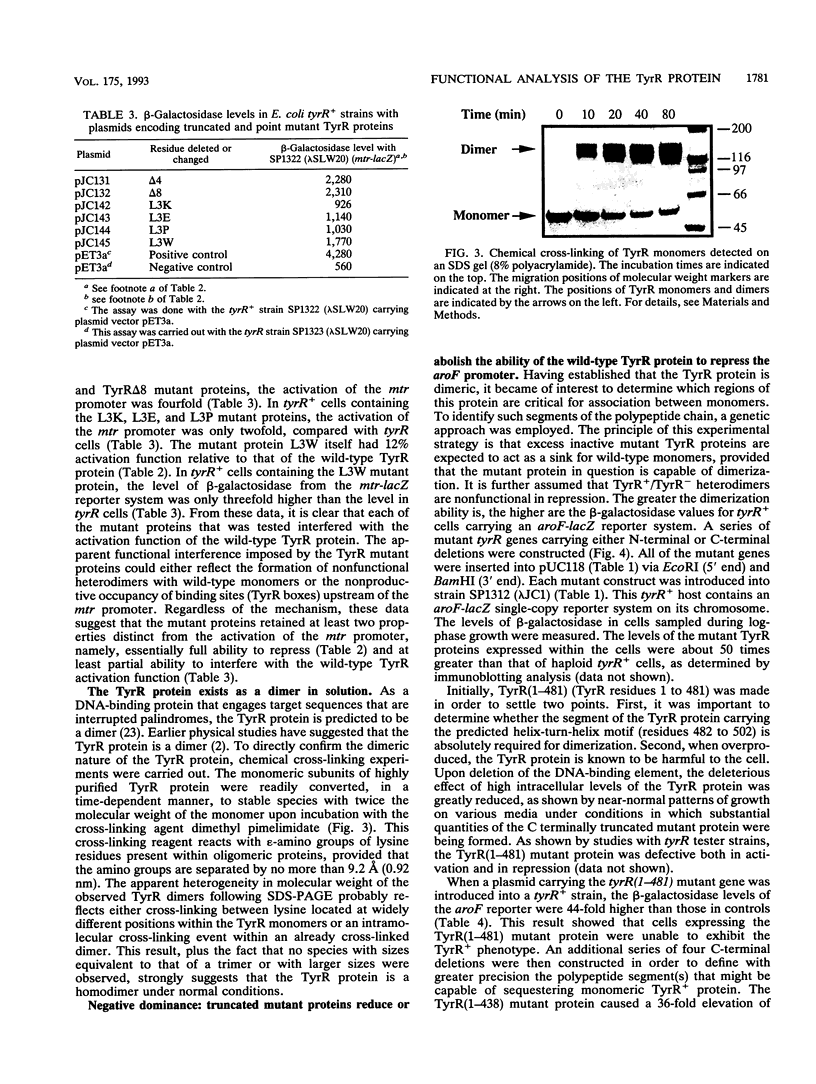
In response to the binding of tyrosine or phenylalanine, the TyrR protein (513 amino acids) activates certain promoters and represses others. In a previous study (J. Cui and R. L. Somerville, J. Bacteriol. 175:303-306, 1993), it was shown that promoter activation was selectively abolished in mutant proteins lacking amino acid residues 2 to 9. An additional series of constructs that encoded mutant TyrR proteins having deletions or point mutations near the N terminus were analyzed. Residues Arg-2 and Leu-3 were shown to be critical for the activation of the mtr promoter. In confirmation of previous findings, none of the activation-defective mutant TyrR proteins had lost significant repression function. The TyrR protein was shown by chemical cross-linking to be dimeric. The polypeptide segments critical for dimer formation in vivo were identified by evaluating the negative dominance phenotypes of a series of mutant proteins, all defective in DNA binding, lacking progressively greater numbers of amino acid residues from either the N terminus or the C terminus. Amino acid residues 194 to 438 were found to contain all of the essential dimerization determinants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews A. E., Dickson B., Lawley B., Cobbett C., Pittard A. J. Importance of the position of TYR R boxes for repression and activation of the tyrP and aroF genes in Escherichia coli. J Bacteriol. 1991 Aug;173(16):5079–5085. doi: 10.1128/jb.173.16.5079-5085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Dixon R. The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J. 1992 Jun;11(6):2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Kundrot C., Dixon R. Influence of a mutation in the putative nucleotide binding site of the nitrogen regulatory protein NTRC on its positive control function. Nucleic Acids Res. 1991 May 11;19(9):2281–2287. doi: 10.1093/nar/19.9.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon W., Buck M. Central domain of the positive control protein NifA and its role in transcriptional activation. J Mol Biol. 1992 May 20;225(2):271–286. doi: 10.1016/0022-2836(92)90921-6. [DOI] [PubMed] [Google Scholar]
- Cobbett C. S., Morrison S., Pittard J. Isolation and analysis of aroFo mutants by using an aroF-lac operon fusion. J Bacteriol. 1984 Jan;157(1):303–310. doi: 10.1128/jb.157.1.303-310.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish E. C., Argyropoulos V. P., Pittard J., Davidson B. E. Structure of the Escherichia coli K12 regulatory gene tyrR. Nucleotide sequence and sites of initiation of transcription and translation. J Biol Chem. 1986 Jan 5;261(1):403–410. [PubMed] [Google Scholar]
- Cui J., Somerville R. L. Mutational uncoupling of the transcriptional activation function of the TyrR protein of Escherichia coli K-12 from the repression function. J Bacteriol. 1993 Jan;175(1):303–306. doi: 10.1128/jb.175.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Somerville R. L. Physical map location and transcriptional orientation of the tyrR gene of Escherichia coli K-12. J Bacteriol. 1992 Jun;174(11):3832–3833. doi: 10.1128/jb.174.11.3832-3833.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Contreras A., Mitchenall L. A. The function of isolated domains and chimaeric proteins constructed from the transcriptional activators NifA and NtrC of Klebsiella pneumoniae. Mol Microbiol. 1990 Jan;4(1):29–37. doi: 10.1111/j.1365-2958.1990.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Heatwole V. M., Somerville R. L. The tryptophan-specific permease gene, mtr, is differentially regulated by the tryptophan and tyrosine repressors in Escherichia coli K-12. J Bacteriol. 1991 Jun;173(11):3601–3604. doi: 10.1128/jb.173.11.3601-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E., Stigter J., Ausubel F. M. The central domain of Rhizobium leguminosarum DctD functions independently to activate transcription. J Bacteriol. 1992 Feb;174(4):1428–1431. doi: 10.1128/jb.174.4.1428-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasian P. A., Davidson B. E., Pittard J. Molecular analysis of the promoter operator region of the Escherichia coli K-12 tyrP gene. J Bacteriol. 1986 Aug;167(2):556–561. doi: 10.1128/jb.167.2.556-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Muday G. K., Johnson D. I., Somerville R. L., Herrmann K. M. The tyrosine repressor negatively regulates aroH expression in Escherichia coli. J Bacteriol. 1991 Jun;173(12):3930–3932. doi: 10.1128/jb.173.12.3930-3932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard A. J., Davidson B. E. TyrR protein of Escherichia coli and its role as repressor and activator. Mol Microbiol. 1991 Jul;5(7):1585–1592. doi: 10.1111/j.1365-2958.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sarsero J. P., Wookey P. J., Pittard A. J. Regulation of expression of the Escherichia coli K-12 mtr gene by TyrR protein and Trp repressor. J Bacteriol. 1991 Jul;173(13):4133–4143. doi: 10.1128/jb.173.13.4133-4143.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville R. L., Shieh T. L., Hagewood B., Cui J. S. Gene expression from multicopy T7 promoter vectors proceeds at single copy rates in the absence of T7 RNA polymerase. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1056–1062. doi: 10.1016/0006-291x(91)92044-k. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Whipp M. J., Pittard A. J. Regulation of aromatic amino acid transport systems in Escherichia coli K-12. J Bacteriol. 1977 Nov;132(2):453–461. doi: 10.1128/jb.132.2.453-461.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]