Abstract
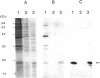
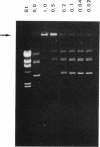
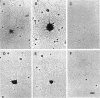
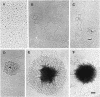
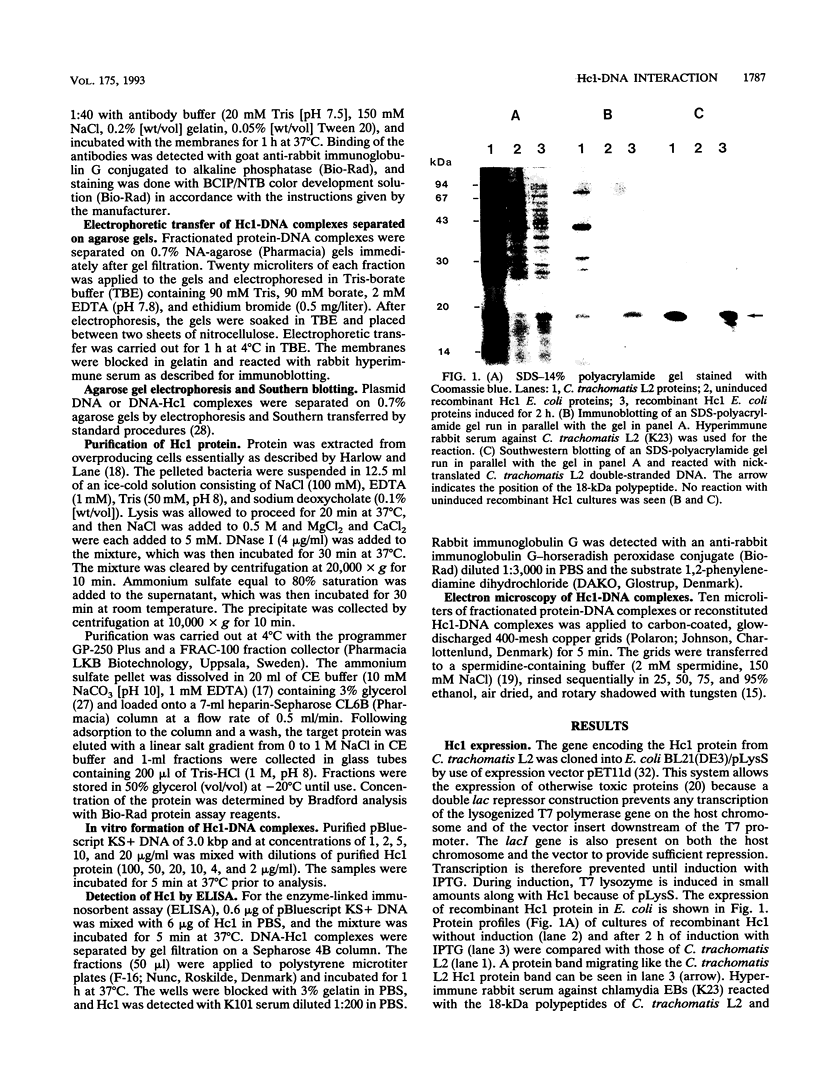
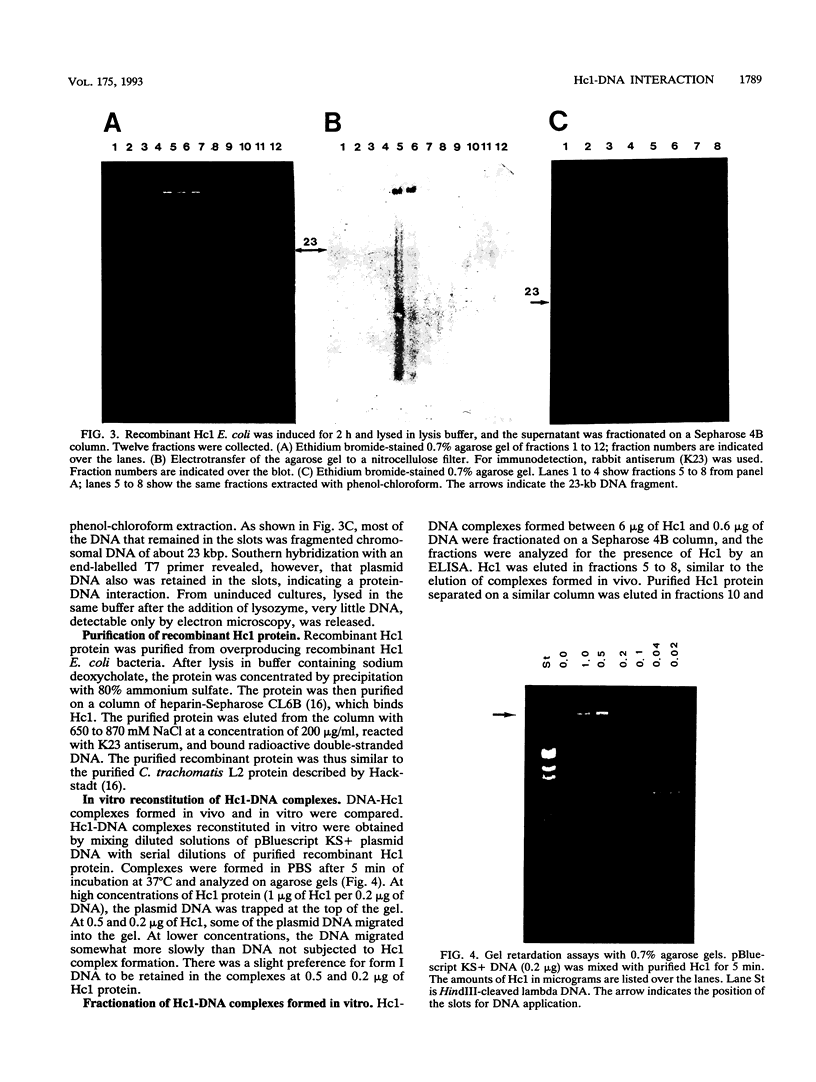
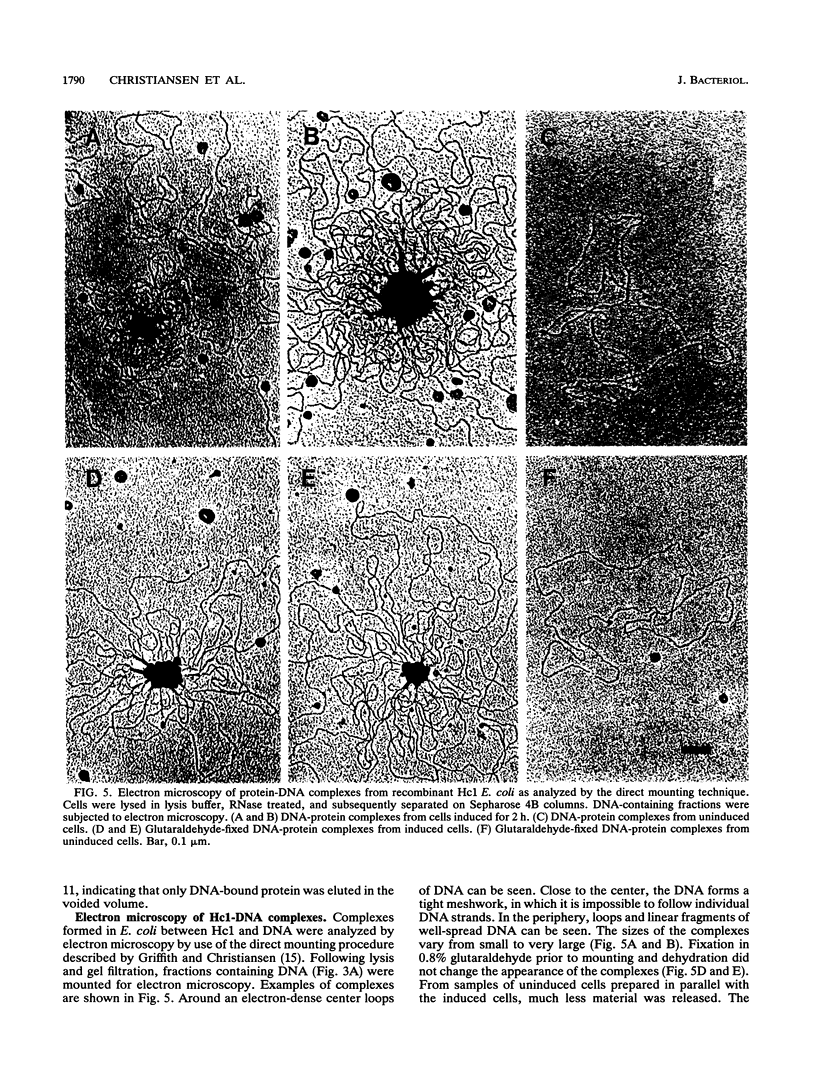
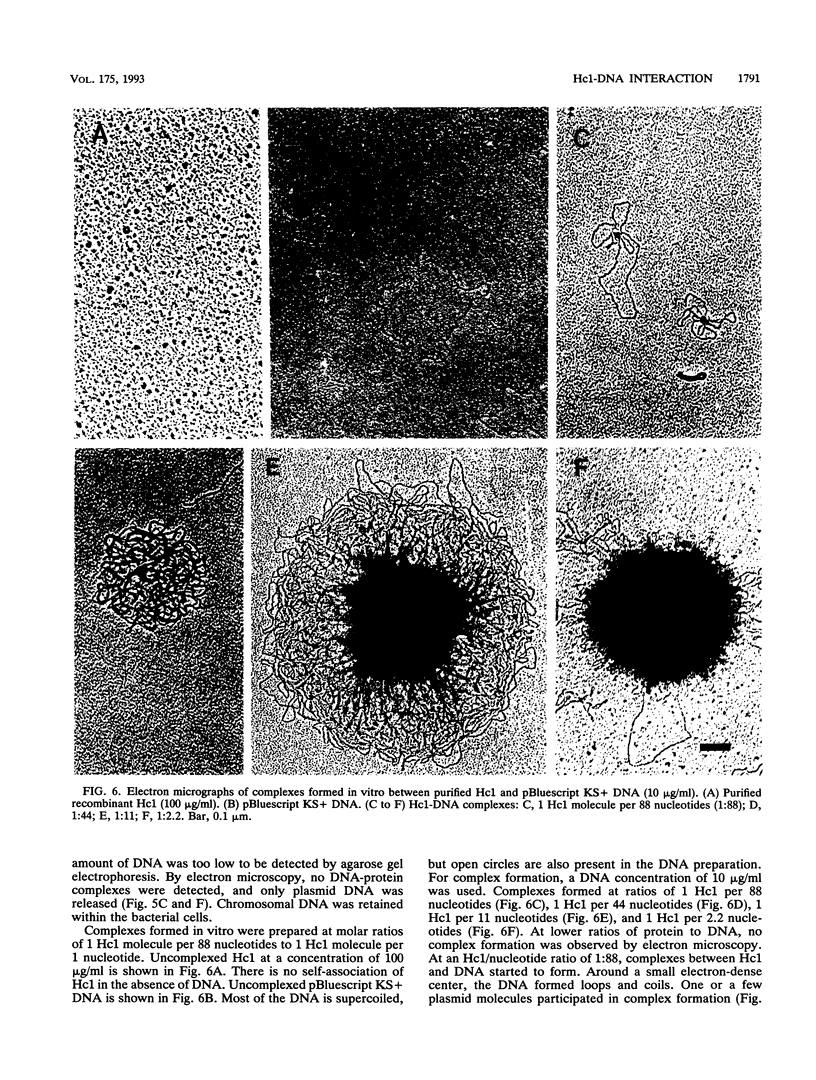
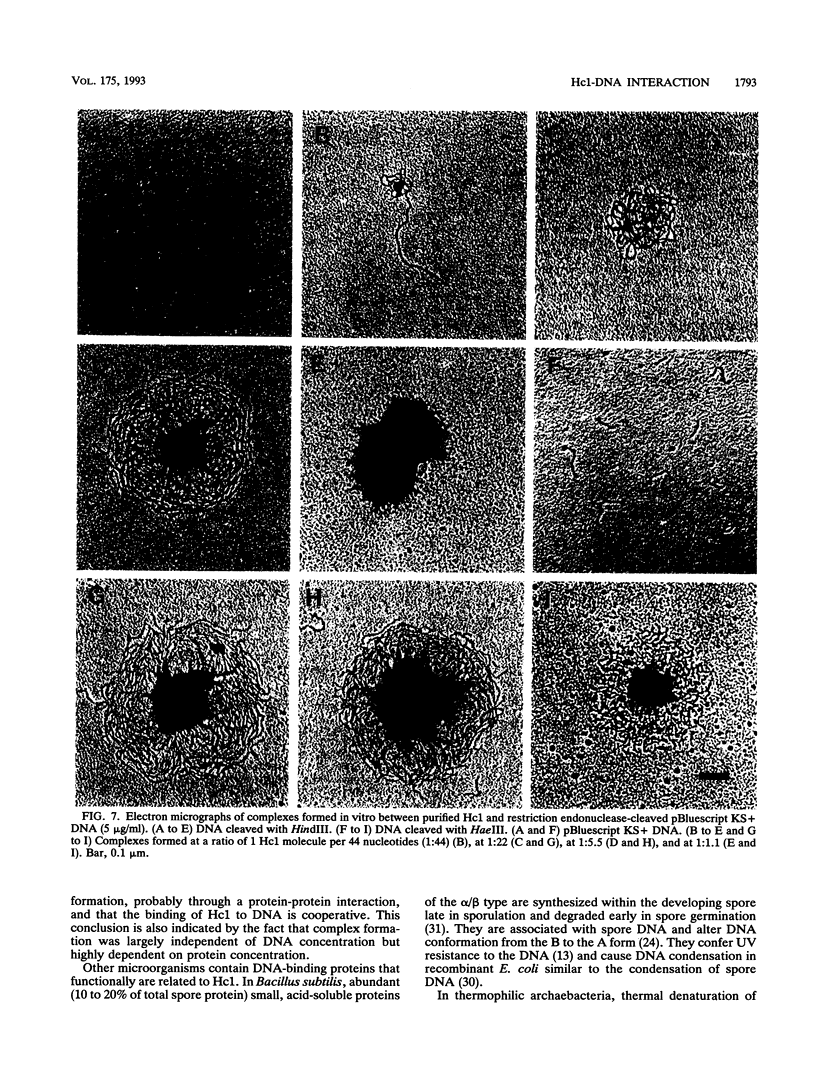
The gene encoding the Chlamydia trachomatis histone H1-like protein (Hc1) from serovar L2 was cloned into Escherichia coli by use of expression vector pET11d. In this vector, transcription of the gene is under the control of a bacteriophage T7 promoter, and T7 RNA polymerase is inducible in the host. Following induction, the E. coli cells were lysed gently. Gel filtration of the lysate revealed comigration of DNA and Hc1 in the voided volume. Electron microscopy revealed the DNA to be complexed with protein in large aggregates, often in the form of spherical bodies. Purified recombinant Hc1 maintained its DNA-binding capacity and was able at high concentrations to form condensed aggregates with DNA (one molecule of Hc1 per base pair) independently of the form or size of the DNA but with a slight preference for supercoiled DNA. Hc1 alone is thus able to package DNA into condensed spherical bodies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Barry C. E., 3rd, Hayes S. F., Hackstadt T. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science. 1992 Apr 17;256(5055):377–379. doi: 10.1126/science.256.5055.377. [DOI] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. Characterization of native and recombinant 75-kilodalton immunogens from Chlamydia trachomatis serovar L2. Infect Immun. 1989 Sep;57(9):2683–2690. doi: 10.1128/iai.57.9.2683-2690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. Chemical cross-linking of Chlamydia trachomatis. Infect Immun. 1988 Mar;56(3):654–659. doi: 10.1128/iai.56.3.654-659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Stephens R. S. Construction of physical and genetic maps of Chlamydia trachomatis serovar L2 by pulsed-field gel electrophoresis. J Bacteriol. 1992 May;174(9):2742–2747. doi: 10.1128/jb.174.9.2742-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J., Thomas J. O. Salt-dependent co-operative interaction of histone H1 with linear DNA. J Mol Biol. 1986 Feb 20;187(4):569–580. doi: 10.1016/0022-2836(86)90335-9. [DOI] [PubMed] [Google Scholar]
- Cole R. D. A minireview of microheterogeneity in H1 histone and its possible significance. Anal Biochem. 1984 Jan;136(1):24–30. doi: 10.1016/0003-2697(84)90303-8. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Poffenroth L., Wilt J. C., Kordová N. Ultrastructural studies of the nucleoids of the pleomorphic forms of Chlamydia psittaci 6BC: a comparison with bacteria. Can J Microbiol. 1976 Jan;22(1):16–28. doi: 10.1139/m76-003. [DOI] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick S., Nicolai M., Mumberg D., Doenecke D. Human H1 histones: conserved and varied sequence elements in two H1 subtype genes. Eur J Cell Biol. 1989 Jun;49(1):110–115. [PubMed] [Google Scholar]
- Fairhead H., Setlow P. Binding of DNA to alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species prevents formation of cytosine dimers, cytosine-thymine dimers, and bipyrimidine photoadducts after UV irradiation. J Bacteriol. 1992 May;174(9):2874–2880. doi: 10.1128/jb.174.9.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D., Christiansen G. Electron microscope visualization of chromatin and other DNA-protein complexes. Annu Rev Biophys Bioeng. 1978;7:19–35. doi: 10.1146/annurev.bb.07.060178.000315. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Baehr W., Ying Y. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3937–3941. doi: 10.1073/pnas.88.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Purification and N-terminal amino acid sequences of Chlamydia trachomatis histone analogs. J Bacteriol. 1991 Nov;173(21):7046–7049. doi: 10.1128/jb.173.21.7046-7049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. H., Griffith J. D. The terminus of SV40 DNA replication and transcription contains a sharp sequence-directed curve. Cell. 1988 Feb 26;52(4):535–544. doi: 10.1016/0092-8674(88)90466-7. [DOI] [PubMed] [Google Scholar]
- Koehler J. E., Birkelund S., Stephens R. S. Overexpression and surface localization of the Chlamydia trachomatis major outer membrane protein in Escherichia coli. Mol Microbiol. 1992 May;6(9):1087–1094. doi: 10.1111/j.1365-2958.1992.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. C., Sokolov N. V., He C. M., Setlow P. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):77–81. doi: 10.1073/pnas.88.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara E., Ganem D., Engel J. N. A developmentally regulated chlamydial gene with apparent homology to eukaryotic histone H1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2125–2129. doi: 10.1073/pnas.89.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A. T., Pérez L., Morán F., Montero F., Suau P. Cooperative interaction of the C-terminal domain of histone H1 with DNA. Biophys Chem. 1991 Feb;39(2):145–152. doi: 10.1016/0301-4622(91)85016-j. [DOI] [PubMed] [Google Scholar]
- Rutberg S. E., Ronai Z. A simple and efficient method for the purification of specific DNA binding proteins. Nucleic Acids Res. 1992 Apr 11;20(7):1815–1815. doi: 10.1093/nar/20.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman K., Krzycki J. A., Dobrinski B., Lurz R., Reeve J. N. HMf, a DNA-binding protein isolated from the hyperthermophilic archaeon Methanothermus fervidus, is most closely related to histones. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5788–5791. doi: 10.1073/pnas.87.15.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Hand A. R., Setlow P. Synthesis of a Bacillus subtilis small, acid-soluble spore protein in Escherichia coli causes cell DNA to assume some characteristics of spore DNA. J Bacteriol. 1991 Mar;173(5):1642–1653. doi: 10.1128/jb.173.5.1642-1653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Rees C., Finch J. T. Cooperative binding of the globular domains of histones H1 and H5 to DNA. Nucleic Acids Res. 1992 Jan 25;20(2):187–194. doi: 10.1093/nar/20.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Stephens R. S. Developmental-form-specific DNA-binding proteins in Chlamydia spp. Infect Immun. 1988 Jul;56(7):1678–1684. doi: 10.1128/iai.56.7.1678-1684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]
- Widom J., Klug A. Structure of the 300A chromatin filament: X-ray diffraction from oriented samples. Cell. 1985 Nov;43(1):207–213. doi: 10.1016/0092-8674(85)90025-x. [DOI] [PubMed] [Google Scholar]