Abstract
We report that temporal spike sequences from hippocampal place neurons of rats on an elevated track recurred in reverse order at the end of a run, but in forward order in anticipation of the run, coinciding with sharp waves. Vector distances between the place fields were reflected in the temporal structure of these sequences. This bidirectional re-enactment of temporal sequences may contribute to the establishment of higher-order associations in episodic memory.
The memory of a temporal sequence is characterized by both forward and backward associations among the stored items of the sequence, with forward associations showing stronger bonds1-3. The storage of the temporal sequence is thought to involve hippocampus-dependent mechanisms during waking and sleep2,4-6. In the hippocampus proper, temporal sequences are observed on several timescales: (i) at the behavioral timescale, as animals run through sequences of place-tuned fields7, (ii) at the timescale of hippocampal theta oscillations, as cells fire with location-dependent phases in a theta cycle (‘phase-precession’)8,9 and (iii) at the timescale of sharp-wave ripples, when large populations of neurons co-fire with fine temporal structure6,10. Here we show that during waking sharp waves in rats, sequences defined at the behavioral timescale, are pre- and replayed, in forward and in reverse, at the beginning and end of a journey, respectively.
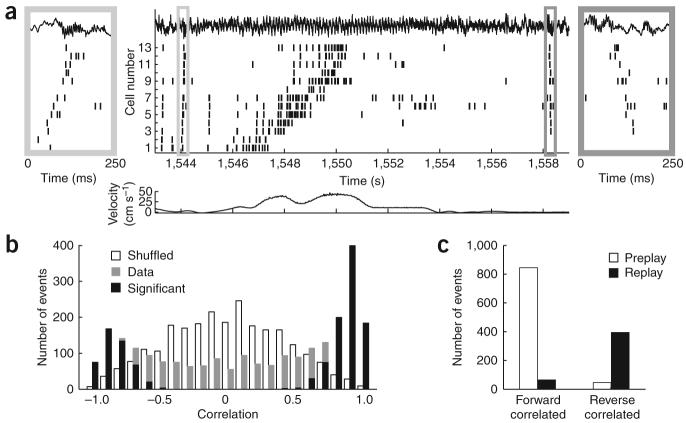
Population activity in the hippocampus was recorded in three rats while they ran back and forth on a linear track for a water reward at each end10 (Fig. 1a). During the run, each neuron's firing was tuned to a particular location along the track, which was stable from lap to lap7. These locations define a temporal sequence of place-cell firing on the timescale of seconds (see Supplementary Methods and Supplementary Figs. 1-10 online). During immobility following the run, the same neurons fired again on the timescale of hundreds of milliseconds, but in the reverse temporal order, confirming previous findings10 (Fig. 1a, right inset). In addition, the neurons fired in the forward temporal order during immobility prior to the run (Fig. 1b, left inset).
Figure 1.
Forward and reverse pre- and replay of place-cell sequences. (a) Spike trains of 13 neurons before, during, and after a single lap (CA1 local field potential shown on top; velocity of the rat shown in the lower panel). The left and right insets magnify 250-ms sections of the spike train, depicting forward preplay and reverse replay, respectively. (b) Histograms show rank-order correlations of the immobility sequences (gray) and an equal number of shuffled surrogate events (white) to the place-field run sequence template. Significantly correlated events are in black. (c) 95% of significant forward correlated events took place before the run (preplay), whereas 85% of significant reverse correlated events took place after the run (replay).
To detect and quantify these ordered events, we treated the left and right laps on the track separately and for each created a place-cell sequence (‘template’) according to the temporal order of peak firing on the track, calculated over the entire session. Laps in different directions generated uniquely different templates, with only 267 out of 1,256 neurons firing ≥5 Hz in both directions11. For each template, we identified reactivation events at both ends of the track by an increase in the population activity during immobility10, when ≥30% or ≥5 of the place cells, whichever was greater, fired in 300-ms windows that were preceded by ≥60-ms silence (sample events are shown in Supplementary Fig. 2). We defined the event sequence on the basis of the firing order of the first spikes from the neurons (see also Supplementary Results online and Supplementary Fig. 10). To test the statistical significance of the temporal ordering within the sequence, we calculated the rank-order correlation between the event sequence and the place-field sequence template. Then for each event, we created 500 surrogate events by shuffling the identity of the neurons (Supplementary Fig. 3). If the event sequence was either more positively or more negatively correlated with the place field sequence template than were 95% of the surrogate event sequences, it was labeled a significant event (Fig. 1b).
Thirty-six percent of all events were significantly forward correlated and 19 percent were significantly reverse correlated, in excess of the number expected by chance (P < 6 × 10−10 forward, P < 3.5 × 10−3 reverse). Thus, approximately twice as many positively (forward) correlated significant events were detected as negatively (reverse) correlated events (1.74 < ratio < 2.11 with 95% confidence). Notably, the majority (841 out of 887, P < 10−190) of positively correlated significant events occurred at the start end of the track before running (preplay), whereas the negatively correlated significant events (395 out of 464; P < 10−57) occurred at the other end following the run (replay; Fig. 1c). Both preplay and replay events were correlated with sharp-wave ripples, which were detected by filtering and thresholding the CA1 local field power in the 100–300-Hz (ripple) band (Fig. 2a and Supplementary Methods).
Figure 2.
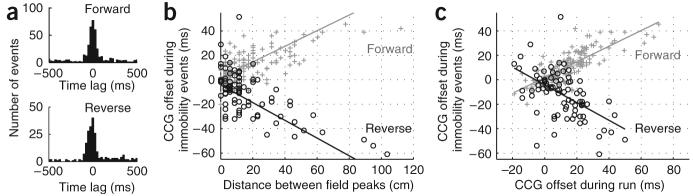
Forward and reverse temporal sequences during ripples reflect distance representations of place cells on the track. (a) Cross-correlograms (CCGs) of forward (preplay) and reverse (replay) events with CA1 sharp-wave ripple events. (b) Temporal offsets between spikes of neuron pairs during significant forward (+) and reverse (o) events were correlated with distance representations between place-field peaks on the track. (c) Temporal offsets of cell pairs were related between track running (‘theta-scale time compression’9) and forward (preplay) and reverse (replay) events.
Beyond preserving temporal order, do the ripple-associated neuronal sequences contain information about the distances between place fields on the track? To address this, we calculated the cross-correlations between cell pairs (within ± 80-ms windows) during running on the track and during ripple-associated immobility events. For pairs that showed a significant peak in the cross-correlation (Supplementary Fig. 5), we compared the time offset of the peak during pre- or replay reactivation epochs with the distance between the place-field peaks on the track (Fig. 2b). Distance information on the track was faithfully preserved in the temporal patterns during preplay (r = 0.66) and replay events (r = −0.66). Next, we examined the temporal relationship of neurons on the theta oscillation timescale during running and during immobility events. We found a strong correlation between the temporal offsets of neuron pairs during forward (preplay) events and theta oscillations during running9 (Fig. 2c; r = 0.80), with data points tightly concentrated around the best fit (R2 = 0.64). The relationship between theta and reverse (replay) events was less correlated (r = −0.54) and the data points were more scattered (R2 = 0.29). These observations suggest that forward preplay may be more directly linked to the cellular representation of the run sequence than is reverse replay.
In the waking animal, two mechanisms appear to favor forward associations: time compression of neuronal sequences within the timescale of theta9,12, and sharp-wave ripples. In addition, reverse replay of run sequences at the end of the trial could facilitate backward associations6,10. A simple place field–based model may account for both forward preplay and reverse replay events (Supplementary Figs. 11 and 12 online). The stronger correlations between place-cell sequences in theta cycles on the track and forward sequences in a larger portion of ripples, together with further strengthening of forward associations during subsequent sleep2,5,13,14, may explain why forward associations are favored as compared with backward associations during free recall1,3. Alternately, preplay events may have a role in ‘planning’ upcoming trajectories15.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank A. Amarasingham and S. Royer for excellent discussions, and S. Montgomery, A. Renart and D. Robbe for comments on the manuscript. This work was supported by the US National Institutes of Health (NS34994 and MH54671).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Kahana MJ. Mem. Cognit. 1996;24:103–109. doi: 10.3758/bf03197276. [DOI] [PubMed] [Google Scholar]
- 2.Drosopoulos S, Windau E, Wagner U, Born J. PLoS ONE. 2007;2:e376. doi: 10.1371/journal.pone.0000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard MW, Fotedar MS, Datey AV, Hasselmo ME. Psychol. Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MA, McNaughton BL. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 6.Buzsaki G. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 7.O'Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 8.O'Keefe J, Recce ML. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 9.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Foster DJ, Wilson MA. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 11.Mehta MR, Barnes CA, McNaughton BL. Proc. Natl. Acad. Sci. USA. 1997;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen O, Lisman JE. J. Neurophysiol. 2000;83:2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- 13.Skaggs WE, McNaughton BL. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 14.Lee AK, Wilson MA. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 15.Ferbinteanu J, Shapiro ML. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.