Abstract
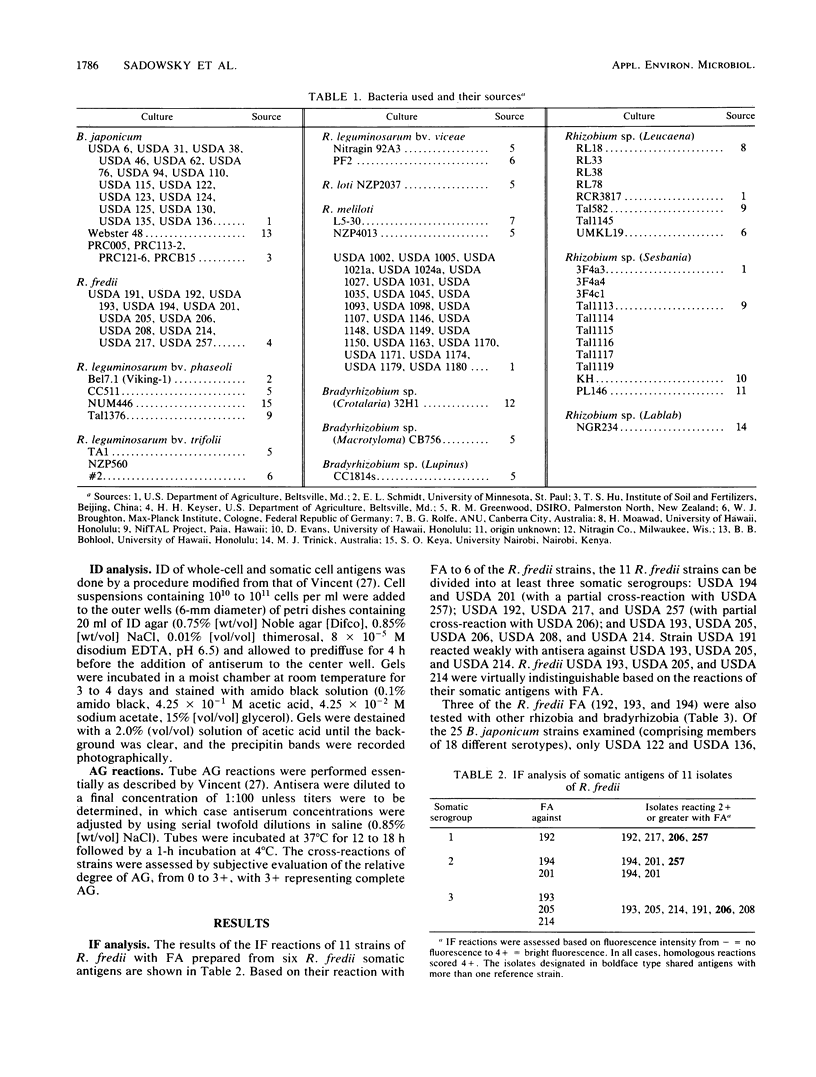
Several isolates of Rhizobium fredii were examined for their serological relatedness to each other, to Bradyrhizobium japonicum, and to other fast- and slow-growing rhizobia. Immunofluorescence, agglutination, and immunodiffusion analyses indicated that R. fredii contains at least three separate somatic serogroups, USDA 192, USDA 194, and USDA 205. There was no cross-reaction between any of the R. fredii isolates and 13 of the 14 B. japonicum somatic serogroups tested. Cross-reactions were obtained with antisera from R. fredii and serogroup 122 of B. japonicum, Rhizobium meliloti, and several fast-growing Rhizobium spp. for Leucaena, Sesbania, and Lablab species. The serological relationship between R. fredii and R. meliloti was examined in more detail, and of 23 R. meliloti strains examined, 8 shared somatic antigens with the type strains from all three R. fredii serogroups. The serological relatedness of R. fredii to B. japonicum and R. meliloti appears to be unique since the strains are known to be biochemically and genetically diverse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Bushnell O. A., Sarles W. B. Investigations upon the Antigenic Relationships among the Root-Nodule Bacteria of the Soybean, Cowpea, and Lupine Cross-Inoculation Groups. J Bacteriol. 1939 Oct;38(4):401–410. doi: 10.1128/jb.38.4.401-410.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATE R. A., DECKER A. M. MINIMAL ANTIGENIC CONSTITUTION OF 28 STRAINS OF RHIZOBIUM JAPONICUM. Can J Microbiol. 1965 Feb;11:1–8. doi: 10.1139/m65-001. [DOI] [PubMed] [Google Scholar]
- DUDMAN W. F. IMMUNE DIFFUSION ANALYSIS OF THE EXTRACELLULAR SOLUBLE ANTIGENS OF TWO STRAINS OF RHIZOBIUM MELILOTI. J Bacteriol. 1964 Sep;88:782–794. doi: 10.1128/jb.88.3.782-794.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle S. F., Bohlool B. B. Predominance of Fast-Growing Rhizobium japonicum in a Soybean Field in the People's Republic of China. Appl Environ Microbiol. 1985 Nov;50(5):1171–1176. doi: 10.1128/aem.50.5.1171-1176.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman W. F. Antigenic analysis of Rhizobium japonicum by immunodiffusion. Appl Microbiol. 1971 Jun;21(6):973–985. doi: 10.1128/am.21.6.973-985.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM P. H. ANTIGENIC AFFINITIES OF THE ROOT-NODULE BACTERIA OF LEGUMES. Antonie Van Leeuwenhoek. 1963;29:281–291. doi: 10.1007/BF02046070. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- MEANS U. M., JOHNSON H. W., DATE R. A. QUICK SEROLOGICAL METHOD OF CLASSIFYING STRAINS OF RHIZOBIUM JAPONICUM IN NODULES. J Bacteriol. 1964 Mar;87:547–553. doi: 10.1128/jb.87.3.547-553.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S. N., Bohlool B. B. Competition Among Rhizobium leguminosarum Strains for Nodulation of Lentils (Lens esculenta). Appl Environ Microbiol. 1983 Mar;45(3):960–965. doi: 10.1128/aem.45.3.960-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst C. E. Some antigenic properties of cultured cell and bacteroid forms of fast- and slow-growing strains of Lotus rhizobia. Microbios. 1979;24(95):19–28. [PubMed] [Google Scholar]
- Sadowsky M. J., Bohlool B. B. Possible involvement of a megaplasmid in nodulation of soybeans by fast-growing rhizobia from china. Appl Environ Microbiol. 1983 Oct;46(4):906–911. doi: 10.1128/aem.46.4.906-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrdleta V. Serological analysis of eleven strains of Rhizobium japonicum. Antonie Van Leeuwenhoek. 1969;35(1):77–83. doi: 10.1007/BF02219118. [DOI] [PubMed] [Google Scholar]