Abstract
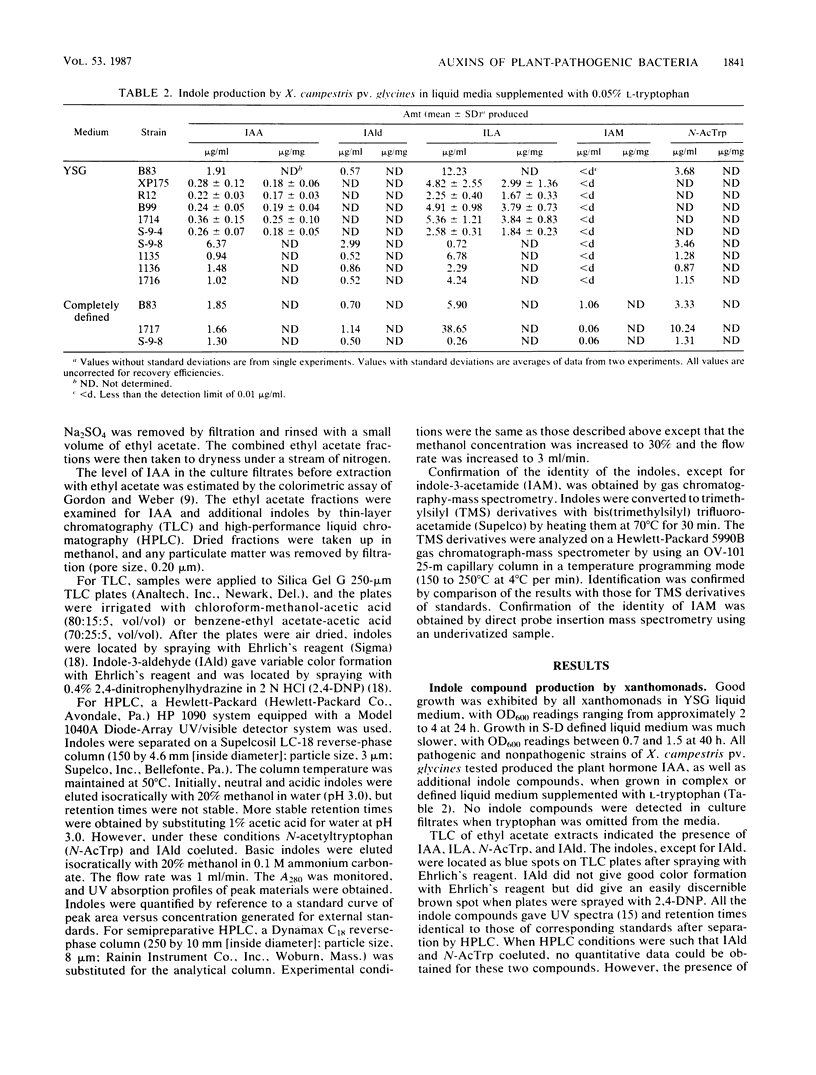
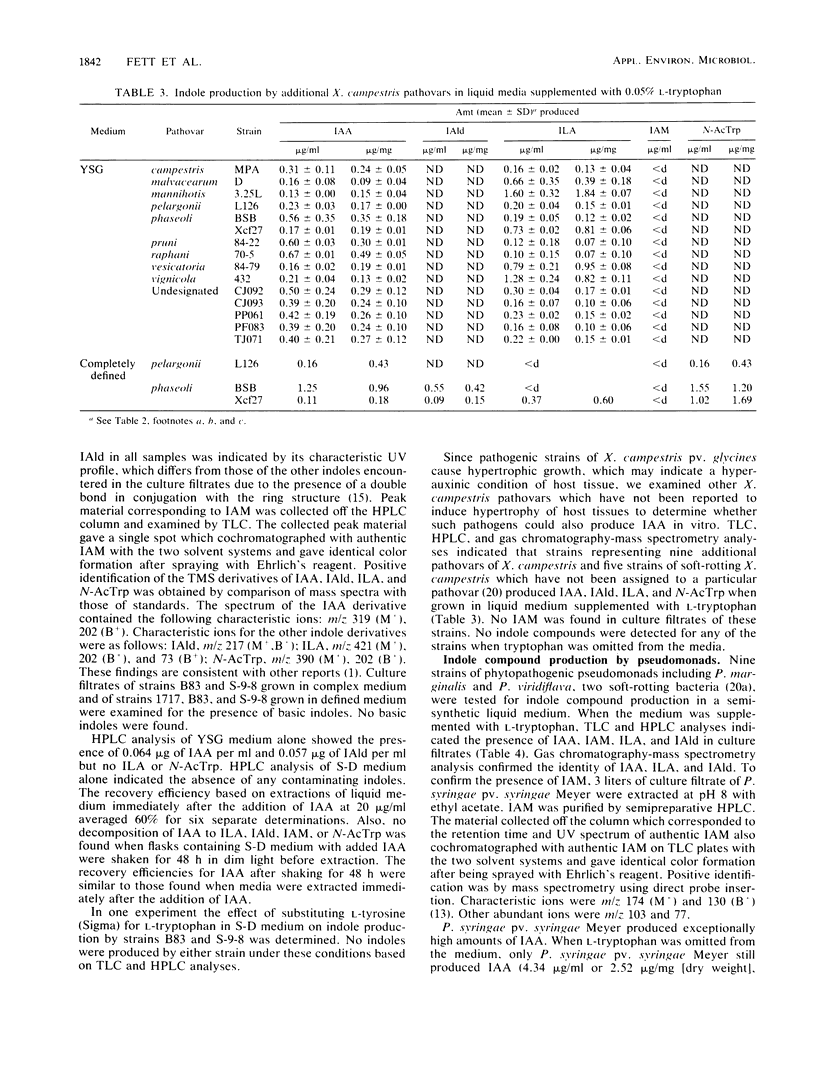
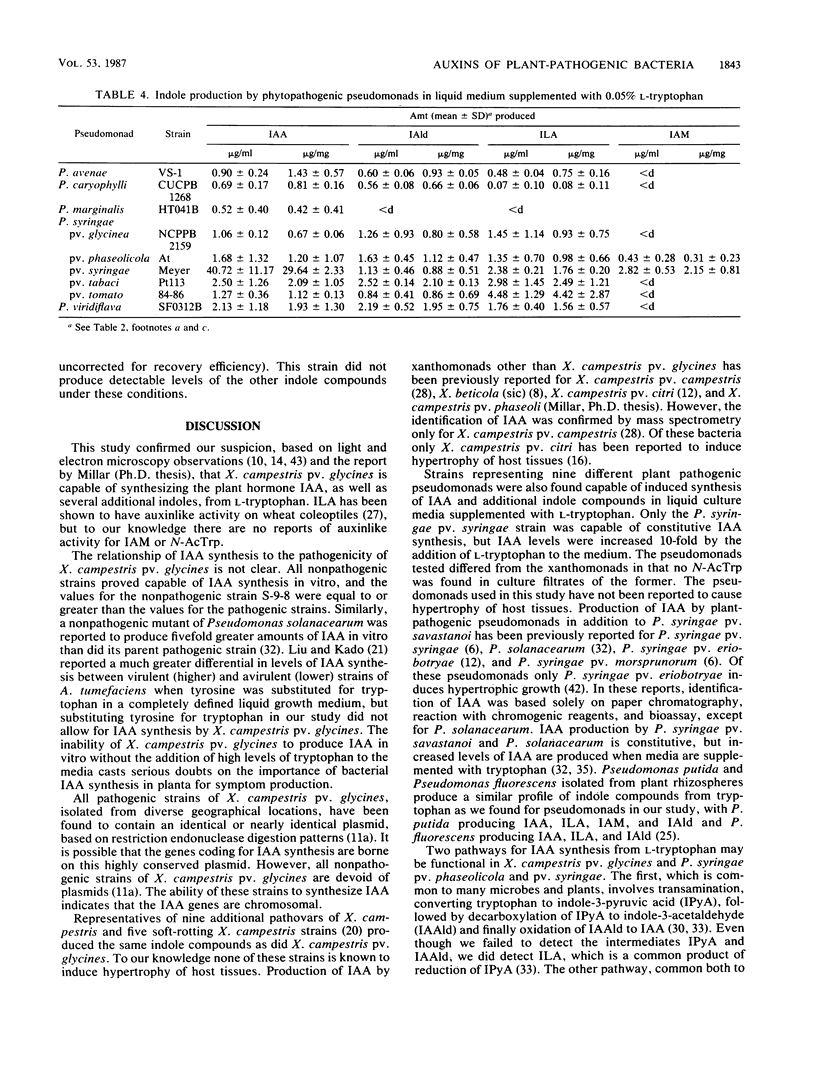
Pathogenic strains of Xanthomonas campestris pv. glycines which cause hypertrophy of leaf cells of susceptible soybean cultivars and nonpathogenic strains which do not cause hypertrophy were compared for their ability to produce indole compounds, including the plant hormone indole-3-acetic acid (IAA) in liquid media with or without supplementation with l-tryptophan. Several additional strains of plant-pathogenic xanthomonads and pseudomonads were also tested for IAA production to determine whether in vitro production of IAA is related to the ability to induce hypertrophic growth of host tissues. Indoles present in culture filtrates were identified by thin-layer chromatography, high-performance liquid chromatography, UV spectroscopy, mass spectroscopy, and gas chromatography-mass spectrometry and were quantitated by high-performance liquid chromatography. All strains examined produced IAA when liquid media were supplemented with l-tryptophan. The highest levels of IAA were found in culture filtrates from the common bean pathogen Pseudomonas syringae pv. syringae, and this was the only bacterium tested which produced IAA without addition of tryptophan to the medium. Additional indoles identified in culture filtrates of the various strains included indole-3-lactic acid, indole-3-aldehyde, indole-3-acetamide, and N-acetyltryptophan. Pseudomonads and xanthomonads could be distinguished by the presence of N-acetyltryptophan, which was found only in xanthomonad culture filtrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barea J. M., Brown M. E. Effects on plant growth produced by Azotobacter paspali related to synthesis of plant growth regulating substances. J Appl Bacteriol. 1974 Dec;37(4):583–593. doi: 10.1111/j.1365-2672.1974.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPER J. M., VELDSTRA H. On the metabolism of tryptophan by Agrobacterium tumefaciens. Biochim Biophys Acta. 1958 Nov;30(2):401–420. doi: 10.1016/0006-3002(58)90065-9. [DOI] [PubMed] [Google Scholar]
- Kosuge T., Heskett M. G., Wilson E. E. Microbial synthesis and degradation of indole-3-acetic acid. I. The conversion of L-tryptophan to indole-3-acetamide by an enzyme system from Pseudomonas savastanoi. J Biol Chem. 1966 Aug 25;241(16):3738–3744. [PubMed] [Google Scholar]
- Liu S. T., Kado C. I. Indoleacetic acid production: a plasmid function of Agrobacterium tumefaciens C58. Biochem Biophys Res Commun. 1979 Sep 12;90(1):171–178. doi: 10.1016/0006-291x(79)91605-x. [DOI] [PubMed] [Google Scholar]
- Liu S. T., Perry K. L., Schardl C. L., Kado C. I. Agrobacterium Ti plasmid indoleacetic acid gene is required for crown gall oncogenesis. Proc Natl Acad Sci U S A. 1982 May;79(9):2812–2816. doi: 10.1073/pnas.79.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offringa I. A., Melchers L. S., Regensburg-Tuink A. J., Costantino P., Schilperoort R. A., Hooykaas P. J. Complementation of Agrobacterium tumefaciens tumor-inducing aux mutants by genes from the T(R)-region of the Ri plasmid of Agrobacterium rhizogenes. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6935–6939. doi: 10.1073/pnas.83.18.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch T., Kahl G., Hilgenberg W. Primary Action of Indole-3-acetic Acid in Crown Gall Tumors: Increase of Solute Uptake. Plant Physiol. 1984 Jun;75(2):354–358. doi: 10.1104/pp.75.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud J. L'acide indolyl-3-lactique et son métabolisme chez Rhizobium. Arch Mikrobiol. 1970;72(4):297–307. [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Souw P., Demain A. L. Nutritional Studies on Xanthan Production by Xanthomonas campestris NRRL B1459. Appl Environ Microbiol. 1979 Jun;37(6):1186–1192. doi: 10.1128/aem.37.6.1186-1192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Hugly S., Buchholz W. G., Thomashow L. S. Molecular basis for the auxin-independent phenotype of crown gall tumor tissues. Science. 1986 Feb 7;231(4738):616–618. doi: 10.1126/science.3511528. [DOI] [PubMed] [Google Scholar]
- Tien T. M., Gaskins M. H., Hubbell D. H. Plant Growth Substances Produced by Azospirillum brasilense and Their Effect on the Growth of Pearl Millet (Pennisetum americanum L.). Appl Environ Microbiol. 1979 May;37(5):1016–1024. doi: 10.1128/aem.37.5.1016-1024.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


