Abstract
Alterations in Hox gene expression patterns have been implicated in both large and small-scale morphological evolution. An improved understanding of these changes requires a detailed understanding of Hox gene cis-regulatory function and evolution. cis-regulatory evolution of the Hox gene Ultrabithorax (Ubx) has been shown to contribute to evolution of trichome patterns on the posterior second femur (T2p) of Drosophila species. As a step toward determining how this function of Ubx has evolved, we performed a series of experiments to clarify the role of Ubx in patterning femurs and to identify the cis-regulatory regions of Ubx that drive expression in T2p. We first performed clonal analysis to further define Ubx function in patterning bristle and trichome patterns in the legs. We found that low levels of Ubx expression are sufficient to repress an eighth bristle row on the posterior second and third femurs, whereas higher levels of expression are required to promote the development and migration of other bristles on the third femur and to repress trichomes. We then tested the hypothesis that the evolutionary difference in T2p trichome patterns due to Ubx was caused by a change in the global cis-regulation of Ubx expression. We found no evidence to support this view, suggesting that the evolved difference in Ubx function reflects evolution of a leg-specific enhancer. We then searched for the regulatory regions of the Ubx locus that drive expression in the second and third femur by assaying all existing regulatory mutations of the Ubx locus and new deficiencies in the large intron of Ubx that we generated by P-element-induced male recombination. We found that two enhancer regions previously known to regulate Ubx expression in the legs, abx and pbx, are required for Ubx expression in the third femur, but that they do not contribute to pupal expression of Ubx in the second femur. This analysis allowed us to rule out at least 100kb of DNA in and around the Ubx locus as containing a T2p-specific enhancer. We then surveyed an additional approximately 30kb using enhancer constructs. None of these enhancer constructs produced an expression pattern similar to Ubx expression in T2p. Thus, after surveying over 95% of the Ubx locus, we have not been able to localize a T2p-specific enhancer. While the enhancer could reside within the small regions we have not surveyed, it is also possible that the enhancer is structurally complex and/or acts only within its native genomic context.
Keywords: cis-regulation, evolution, Drosophila, leg, trichome, Ultrabithorax
INTRODUCTION
Although it has been inferred that much of developmental evolution occurs by changes in cis-regulatory regions, in only a few cases have the individual regulatory changes been identified (Gompel et al., 2005; Wang and Chamberlin, 2004). We have previously shown that the detailed pattern of trichomes on the legs of D. melanogaster is evolving rapidly and that some of this evolutionary change is attributable to cis-regulatory evolution at the Ubx locus (Stern, 1998). A stronger test of this hypothesis, and a more comprehensive understanding of how Ubx has evolved to alter body plans (Averof and Patel, 1997; Mahfooz et al., 2004), requires a detailed understanding of Ubx cis-regulatory structure and evolution.
The three pairs of legs of the adult Drosophila melanogaster differ in size and shape and in the distribution of different types of bristles and trichomes (Hannah-Alava, 1958). These differences are generated, ultimately, by the action of the homeotic genes Sex Combs Reduced (Scr) and Ultrabithorax (Ubx). Scr expression in the prothoracic legs (hereafter T1) is required to define specific features of these legs, such as the sex combs in males, lateral rows of bristles on the anterior tibia, and large bristles on the posterior femur (Struhl, 1982). Ubx function is required to define features of the metathoracic (T3) legs (Casanova et al., 1985; Kerridge and Morata, 1982; Morata and Kerridge, 1981; Struhl, 1982). Ubx clones induced at approximately four hours of development, however, cause a transformation of posterior femurs of both T2 and T3 towards T1, a phenotype known as a postprothorax (ppx) transformation (Casanova et al., 1985; Kerridge and Morata, 1982; Morata and Kerridge, 1981). This effect is due to a loss of Scr repression by Ubx in the posterior of T2 and T3 early in development (Little et al., 1990; Struhl, 1982). Although loss of function clones of Ubx and Scr induced after embryogenesis have no obvious effects on mesothoracic (T2) bristle patterns (Kerridge and Morata, 1982; Morata and Garcia-Bellido, 1976; Morata and Kerridge, 1981; Struhl, 1982), Ubx is required to repress the differentiation of trichomes in a proximal patch of cuticle on the posterior femur of T2 during metamorphosis (Stern, 1998). The size of this patch of smooth or naked cuticle has evolved amongst Drosophila species, with certain strains of D. melanogaster exhibiting a smaller patch relative to D. simulans (Stern, 1998).
Here we present results from a series of experiments that examine the role of Ubx in patterning legs and that seek to identify sequences responsible for differences in Ubx expression that account for an evolved leg phenotype between Drosophila species. We expanded on previous studies and found that in addition to the well characterized functions of Ubx in patterning T3, Ubx function is required to determine the fate and behavior of several cell types in T2 and T3 at multiple times during development. Some functions require high levels of Ubx expression, whereas only low levels of Ubx expression are required for other functions. Analysis of Ubx mutations identifies two previously characterized enhancers as required for Ubx expression in the third femur. To determine how Ubx leg expression has evolved, we first tested for, but found no evidence that, global cis-regulation of Ubx expression has evolved between D. melanogaster and D. simulans. We also found no direct evidence, either through analysis of mutants or analysis of enhancer constructs, for a discrete enhancer responsible for Ubx expression in the second femur. These results point toward complex regulation of Ubx function in T2.
MATERIALS AND METHODS
Ubx clones
Clonal analysis using a null allele of Ubx was performed to provide a detailed characterization of the role of Ubx in patterning trichomes and bristles on T2 and T3 legs. The Minute technique (Morata and Ripoll, 1975) was utilized to generate large territories of tissue homozygous for a Ubx null allele. With this technique, clones that carry two wild-type alleles of Minute+ overproliferate relative to neighboring cells that are heterozygous for a null allele of Minute. (Unless otherwise specified, null alleles are indicated generically with a superscript minus sign and wild-type alleles with a superscript plus sign. For example, clones carrying two wild-type copies of Minute are referred to as Minute+ clones.) Virgin females with the genotype f36a; f+87D M(3)95A/TM3 were crossed to males with the genotype Ubx1e11/TM6B, Tb. (The Ubx1e11 chromosome had previously been outcrossed for several generations to a wild-type chromosome.) Larvae from this cross were irradiated (1,000 rad X-rays) at 24–48 hours after egg-laying (AEL) or 48–72 hours AEL. Early clones were generated by X-ray irradiating embryos at 4±2 hours AEL with 700 rad. Male offspring without balancer chromosomes were then selected and preserved in 70% ethanol:30% glycerol. Control clones were generated by crossing virgin females with the genotype f36a; f+87D M(3)95A/TM3 to males of the genotype st1ppe11. Larvae were irradiated as above and males without the TM3 balancer chromosome were collected. Both experimental and control clones were therefore marked with bristles that were both forked and ebony. Clones were identified by inspecting the legs of flies for forked bristles under a dissecting microscope and all legs from an individual with at least a single clone were then mounted on glass slides in Hoyer’s medium (Stern and Sucena, 2000). Images of adult legs were captured with a video camera under darkfield illumination and digitally inverted.
Ubx+ overexpression from a heat-shock inducible construct HSUbx-1a (Mann and Hogness, 1990) represses trichomes on the posterior T2 and T3 legs from approximately 20–30 hours APF at 25°C (Stern, 1998). We have extended this analysis by performing overexpression studies in flies also carrying an engrailed reporter construct (P{ry+t7.2=en-lacZ(Xho)}enXho25). White pre-pupae (0±0.5 hours APF) from the cross of HSUbx/TM3 to en-lacZ/CyO were aged for 24 hours at 25°C and then heat shocked at 37°C for 1 hour. Pharate adults were dissected from the puparium, fixed in 2.5% glutaraldehyde in Phosphate-buffered saline (PBS: 130mM NaCl; 7mM Na2HPO4.2H2O; 3mM NaH2PO4.2H2O; pH 7.0) for 5 min, and stained for β-galactosidase activity using standard techniques (Ashburner, 1989). After staining, legs were dissected and mounted in Hoyer’s medium (Stern and Sucena, 2000).
The patterns of trichomes and bristles on T1, T2, and T3 femurs possessing Ubx− clones were inspected in detail and compared with control clones and wild-type legs.
Pyrosequencing
Whole pupae of D. melanogaster (Oregon R) (14 total), D. simulans (Tsimbazaza) (14 total) and female hybrids created by mating D. melanogaster females and D. simulans males (6 collections of 14 pupae each) were collected at 24.5 hours after puparium formation (APF) and flash-frozen in liquid nitrogen. Genomic DNA and total RNA were isolated from these collections and single-stranded cDNA was synthesized twice for each RNA sample (Wittkopp et al., 2004). An amplicon within the Ubx homeobox that contains a single-nucleotide difference between D. melanogaster and D. simulans was amplified from gDNA and cDNA samples (primers: 5′-biotin-ATACACCCGCTACCAGACGCTC-3′ and 5′-TTCTCCGTCTGCGGGTCA-3′; region containing mel/sim difference: 5′-AAGGAGTTCCACACGAATCAT/CTAT-3′) and subsequently Pyrosequenced in the region containing the interspecific difference (Pyrosequencing primer: 5′-AGGAGTTCCACACGAATC-3′). The relative amounts of D. melanogaster and D. simulans Ubx mRNA represented in D. melanogaster/D. simulans hybrid cDNA were then determined by comparing the average percent melanogaster allele in PCR product amplified from hybrid cDNA with product amplified from hybrid gDNA, with any deviation from 50% in the latter representing allele-specific PCR amplification bias (Wittkopp et al., 2004).
Ubx mutants and genetic crosses
Stocks carrying mutations in the Ubx locus were provided by the Bloomington, Umea and Madrid Stock Centers, Michael Akam, Ed Lewis, and Welcome Bender. A full list of the alleles used is available in Supplementary Table 1. All regulatory mutations were crossed to at least one deficiency covering the entire Ubx locus (Df(3)P2, Df(3)P9, or Df(3)Ubx109). Legs were dissected and mounted in Hoyer’s medium and trichome and bristle patterns were scored under dark-field and bright-field illumination, respectively (Stern and Sucena, 2000). The distribution of trichomes on the posterior T2 and T3 femur was sketched for all genotypes, and we paid particular attention to similarities between T2 and T3 of individual genotypes and to genotypes that removed most or all of the naked cuticle on the posterior T2. Bristles that distinguish the T2 and T3 legs can be found at many positions along the leg (Hannah-Alava, 1958). The presence of ectopic bristles on the posterior T2 and T3 femur were noted and for some genotypes the number of rows of bristles and total number of bristles on the entire femur were counted. Casanova et al. (Casanova et al., 1985) and Peifer and Bender (Peifer and Bender, 1986) have previously reported that the abx and some of the bx mutations in trans to a deficiency for Ubx generate the ppx transformation with low penetrance. We therefore examine the T2 femurs of abx mutations for ppx transformations.
To mark the compartment boundary in pbx hemizygote flies, we generated flies heterozygous for en-lacZ on the second chromosome and a pbx mutation on the third and crossed these flies either to Df(3)Ubx109/TM6B,Tb or to Df(3)P2/TM6B,Tb. Non-Tubby pupae from this cross were collected at approximately 48–72 hours APF, fixed and stained for β-galactosidase activity as detailed above. The legs from flies simultaneously displaying a pbx haltere phenotype and expressing en-lacZ were mounted in Hoyer’s medium for examination.
Generation and analysis of new deficiencies
We generated new deficiencies at the Ubx locus targeted to the third exon of Ubx by P-element-induced male recombination (Preston et al., 1996) using the rosy+-containing P-element insertion plac(−61) located within the third intron of Ubx (McCall et al., 1994). ry sbd1 3ry138 e11/TM6B males were mated to Δ2–3 cv v/FM7c virgins and the resulting Δ2–3 cv v; ry sbd1 3ry138 e11/+ males were mated to sbd2 ell virgins. Male progeny were then screened for recombinants (absence of sbd− or e− phenotypes). Deficiencies among recombinants were initially identified by failure to PCR-amplify DNA just 5′ and 3′ of plac(−61). Excision of the P element in some recombinants was determined by loss of ry+ eye phenotype when tested in a ry− background and by PCR assays. Breakpoints were determined by inverse PCR when it was determined that the P-element had been retained and by Southern analysis when it had been excised.
Enhancer constructs
Enhancer constructs were derived from a previously cloned library of genomic fragments (Bender et al., 1983). The genomic fragments 3109, 3128, 3130, and 3142 were subcloned into pBluescript SKII+ (Stratagene) and then subcloned again into the Gal4 P-element w+ vector pPTGAL with an hsp70 minimal promoter (Sharma et al., 2002). DNA preparations of these constructs were then injected into w118 embryos at the Duke Model System Genomics Group (http://www.biology.duke.edu/model-system/). Transformants (3 lines of 3109, 1 line of 3128, 2 lines of 3130 and 2 lines of 3142), were crossed to flies carrying UAS-lacZ4-1.2 and the resulting progeny examined by anti-β-galactosidase (Capel) staining for embryos and by X-GAL staining for 3rd instar imaginal discs and pupal legs (22–26 hours APF). An internal PCR product (3118int; primers: 5′-GCAATGTAAGCCCTGTTCGTATCTC -3′ and 5′-CCTAAGTAATGGACGCAACTTCAGG -3′) from genomic fragment 3118 was cloned into the TOPO-TA vector pCR-II (Invitrogen) and then subcloned into the nuclear enhanced GFP P-element w+ vector pH-Stinger with hsp70 minimal promoter and flanking gypsy insulating elements (Barolo et al., 2000). Likewise, an ~3.2 kb 5′ of the first exon (5′ Ubx) was PCR-amplified from genomic OreR DNA (primers: 5′-AGCGGCCGCGAGGGCGTTGAGATAGGCCCCTTCA -3′ (NotI site added at 5′ end) and 5′-AAGATCTCGCGCCTGTTATCCAATCCGTTGC -3′) and cloned into pH-Stinger. The embryos, 3rd instar imaginal discs, and pupal legs of transformants (1 line each for 3118int and 5′ Ubx) were examined by fluorescence microscopy.
RESULTS
Ubx expression is required for specification, repression and migration of different bristles
We first performed clonal analysis to further define Ubx requirements in leg patterning. Our analysis of Ubx− clones agrees with the findings of Kerridge and Morata (1982) that on the T3 leg, Ubx+ activity cell-autonomously represses bristles on the posterior femur (Fig. 2a). We interpreted these bristles as representing an ectopic eighth bristle row, located laterally on the posterior femur, although the bristles were never found in a neat row in clones, as they are elsewhere on the leg. The fine bristles normally located proximal-ventrally on T3p (arrow in Fig. 1d) were lost in posterior Ubx− clones (Fig. 2a), indicating that Ubx+ activity is required to specify these bristles.
Fig. 2.
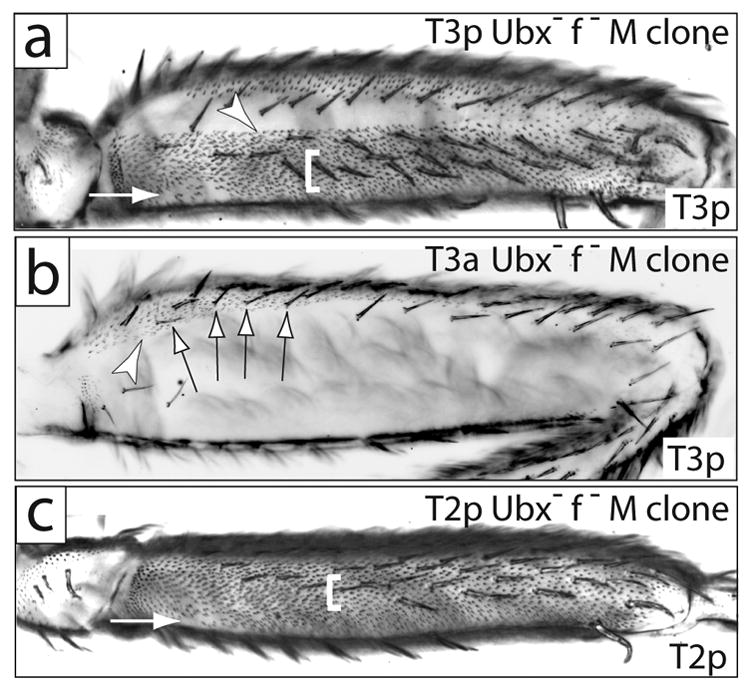
Ubx+ represses most of the trichomes and one lateral bristle row on posterior T2 and T3. (a) Posterior of a T3 femur carrying a Minute+ Ubx− clone that fills the posterior compartment. A small patch of naked cuticle is found proximal-ventrally (arrow). Trichomes differentiate throughout the rest of the posterior compartment. Naked cuticle in the anterior compartment (dorsally) is unaffected. The fine bristles normally found in the proximal lateral region are repressed, and the dorsal bristles are apparently transformed into more robust bristles. Finally, an ectopic, eighth row of bristles differentiates laterally (white bracket). All Minute+ Ubx− clones found on the posterior T3 exhibited a sharp dorsal boundary between derepressed trichomes and naked cuticle (arrowhead). (b) A view of the posterior and dorsal anterior of a femur with a Minute+ Ubx− clone that fills the anterior compartment. Trichomes differentiate in the normally naked cuticle region of the anterior dorsal femur (arrowhead). The small bristles in the dorsal posterior compartment are unaffected by the clone and are forked+, indicating that they originated in the posterior compartment (arrows). (c) T2 posterior femur carrying a Minute+ Ubx− clone that fills the posterior compartment. Trichomes differentiate throughout most of the posterior femur except for a small patch of cuticle proximal-ventrally (arrow). An ectopic, eighth row of bristles is indicated with a white bracket. Clones in (a) and (c) are marked with forked bristles both dorsal and ventral to the small ventral naked patches.
Fig. 1.
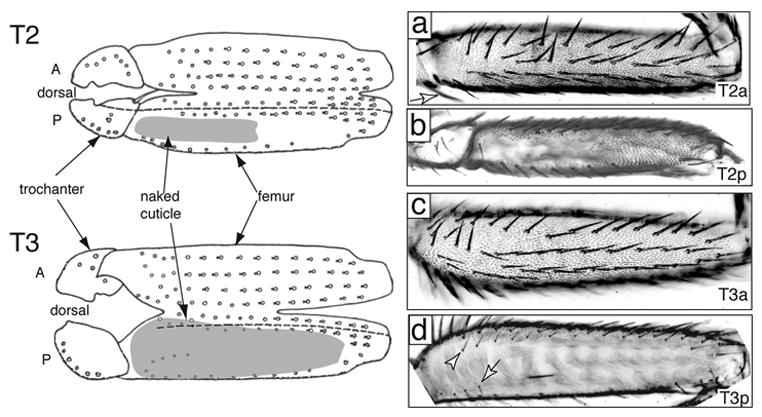
Wildtype mesothoracic (T2) and metathoracic (T3) femurs. A diagrammatic representation of the legs is shown to the left, with the legs split open along the ventral boundary of the anterior/posterior compartment (modified from Steiner 1976). Anterior and posterior compartments are labelled with A and P and are separated by a dashed line. Regions of naked cuticle are shaded gray. The photographs are oriented in the same way as the diagram: in (a) and (c) ventral is up and in (b) and (d) dorsal is up. (A) Anterior T2 femur has five rows of bristles and is completely covered in trichomes. (B) Posterior T2 has one row of bristles dorsally and one ventral row that is incomplete distally. A proximal lateral patch of naked cuticle varies in size between different strains. The remainder of the posterior surface is covered with trichomes. (C) Anterior T3 has five rows of bristles and is covered in trichomes over all but a dorsal proximal region (see D). (D) Posterior T3 has one row of bristles ventrally, several fine bristles in the proximal region (arrow), and a dorsal row of small bristles. Most of the surface is naked, and a small distal region normally produces trichomes. The dorsal anterior-posterior compartment boundary lies approximately along the dorsal bristle row (arrowhead) and does not reflect an obvious morphological discontinuity. The dorsal boundary between trichomes and naked cuticle extends into the proximal anterior dorsal region.
In posterior Ubx− clones, the small bristles normally found dorsally (arrowhead in Fig. 1d) were apparently transformed into more robust bristles (Fig. 2a). In contrast, in anterior Ubx− clones these small bristles remain on T3 (arrows in Fig. 2b) indicating that Ubx+ is required to instruct these bristles to be small. In addition, as will be made clear in subsequent sections, the boundary between naked cuticle and trichomes in the Ubx− clones in Figures 2a and 2b represents the dorsal anterior-posterior boundary. Thus, the small dorsal-proximal bristles are found in the anterior compartment in legs with an anterior Ubx− clone (Fig. 2b), but not in legs with a posterior Ubx− clone (Fig. 2a). In Fig. 2b, it can be seen that these small bristles are not forked−, indicating that they originated in the posterior compartment. This was also observed with control clones (data not shown). These results indicate that Ubx+ expression in the posterior is required for these bristles to migrate from the posterior to the anterior compartment.
Finally, we found that Ubx− clones derepressed a bristle row on the posterior T2 femur (Fig. 2c), as they did on T3, leading to a total of eight bristle rows on the entire femur. In contrast, Kerridge and Morata (1982) reported that the bristle pattern on the posterior T2 femur was unaltered in Ubx− clones generated in the larval period (they found that blastoderm clones generated a ppx transformation). This observation led them to suggest that loss of Ubx+ in the posterior T3 femur generated a homeotic transformation towards T2. Our results suggest, instead, that loss of Ubx+ has the same effect in both legs: gain of an eighth bristle row.
Ubx expression is required to repress most trichomes in the posterior femur and a subset of anterior trichomes
In wild-type legs, the proximal region of the posterior femur of T2 (Fig. 1b) and most of the posterior femur of T3 (Fig. 1d) secrete smooth cuticle. In addition, as will be discussed in more detail, the proximal dorsal anterior T3 femur is also naked (Fig. 1d). Previous studies indicated that Ubx+ represses trichome differentiation on the posterior femur of both T2 and T3 (Kerridge and Morata, 1982; Stern, 1998). Our analysis extends these results. First, large clones in the posterior T2 and T3 derepress trichomes over most, but not all of the posterior femur (Fig. 2a,c). On both legs, clones fail to differentiate trichomes in a small patch on the proximal, ventral region of the posterior femur (arrows in Fig. 2a,c).
In addition, Ubx+ represses trichomes on the dorsal anterior proximal femur of T3 (arrowhead in Fig. 2b), a region that is normally naked (arrowhead in Fig. 1d). Large Minute+ Ubx− clones on T3p leave a large region of the dorsal T3 femur naked (Fig. 2a). This result was found in all legs carrying large Minute+ Ubx− clones on T3p induced between 24–48 AEL (N=9) and between 48–72 AEL (N=7). In contrast, small Ubx− clones induced between 24–48 AEL using the FLP-FRT system (Xu and Rubin, 1993) could be found in the dorsal region and differentiated trichomes (data not shown). This naked dorsal region in large clones is interpreted as the naked cuticle of the anterior compartment that has shifted laterally, and perhaps expanded, because the posterior compartment has shrunk.
The difference between the response of trichome-forming cells in the anterior and posterior compartments to Ubx expression that was reported earlier (Stern, 1998) can now be extended to the differential response of subsets of cells within each compartment. In the posterior compartment of the T2 and T3 femur, all cells except a small patch of proximal ventral cells are capable of responding to high levels of Ubx+ 20–30H APF by repressing trichomes (Stern, 1998); the proximal ventral cells repress trichomes independently of autonomous Ubx+ activity. In the T2 and T3 anterior femur, only cells in the proximal dorsal region are capable of responding to high levels of Ubx+ by repressing trichomes (also see Fig. 5).
Fig. 5.
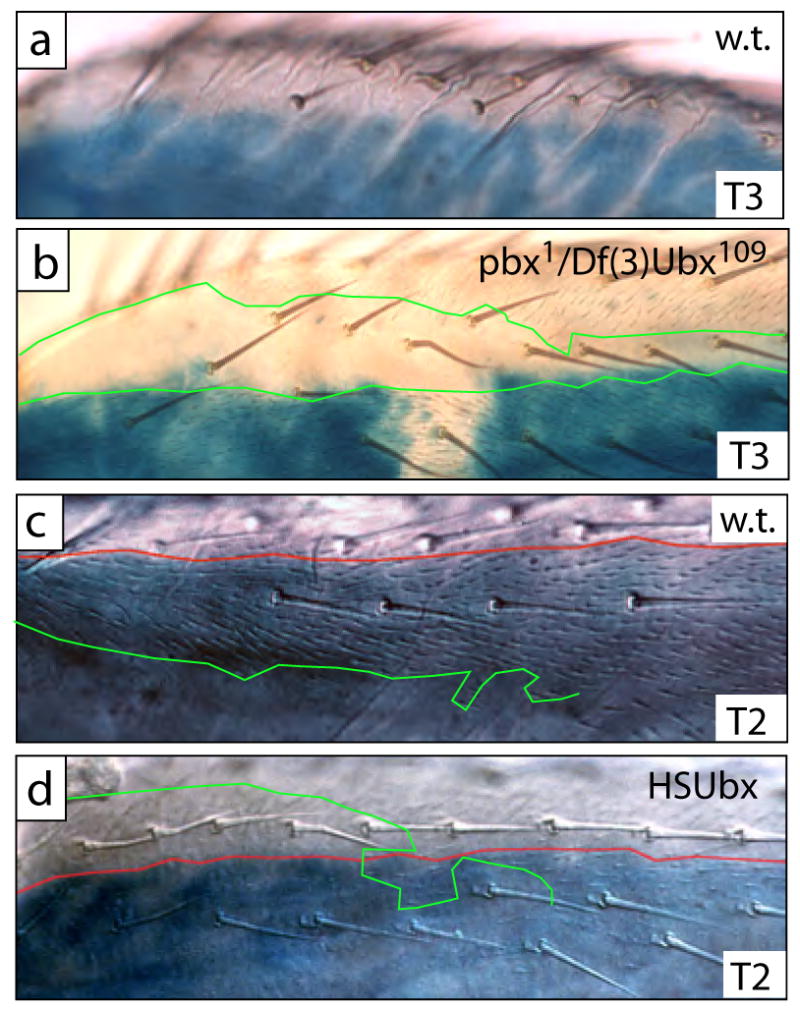
The boundary of naked cuticle and trichomes does not obey the compartment boundary in T2 or T3, and the proximal dorsal anterior femur of both T2 and T3 is competent to repress trichomes when high levels of Ubx are expressed in these cells. (a) In proximal dorsal regions of wild-type T3, naked cuticle is found in both posterior and anterior compartments. Blue en-lacZ staining marks the posterior compartment. (b) In a pbx1 hemizygote naked cuticle is still found in the anterior compartment, but cells in the posterior compartment differentiate trichomes. In this preparation, en-lacZ staining failed in a small patch of cells, revealing the faint trichomes. In most specimens this boundary of en-lacZ staining is complete and approximately straight, and the ectopic trichomes are only found within the region of en-lacZ staining. The boundaries of naked cuticle and trichomes are marked with green lines. (c) In dorsal proximal regions of wild-type T2 legs trichomes differentiate in both the anterior and posterior compartment. The boundary of en-lacZ staining is marked with a red line, and the boundary of trichomes is marked with a green line. (d) When Ubx is expressed ectopically at high levels at 24H APF, cells in this dorsal region now differentiate naked cuticle in both the posterior and anterior compartments. The boundary of en-lacZ staining is marked with a red line, and the border of naked cuticle is marked with a green line.
Global cis-regulation of Ubx expression has not evolved between D. melanogaster and D. simulans
To test the hypothesis that the reduction in the size of the naked patch due to Ubx resulted from a decrease in the global (that is, non-tissue specific) cis-regulation of Ubx in D. melanogaster, we assayed the relative amounts of D. melanogaster and D. simulans Ubx mRNA in whole pupae of D. melanogaster/D. simulans hybrids by Pyrosequencing (Wittkopp et al., 2004) at the time when trichomes are repressed on T2p. We found that the percent melanogaster allele in PCR product amplified from hybrid cDNA was 48.9% (SD = 6.2%) and from hybrid gDNA was 47.6% (SD = 0.3%), which is not significantly different (two-tailed t-test, assuming unequal variances, t=−0.512, df = 5, p = 0.63). The variability observed among replicate measures of Ubx expression was larger than the variability observed for other genes using the same technique (Landry et al., 2005; Wittkopp et al., 2004; Wittkopp et al., 2006) and is likely due to the rarity of Ubx transcripts versus the relative abundance of previously surveyed mRNA. We attempted to measure the relative levels of mRNA in hybrid pupal legs, but the signal to noise ratio was not sufficiently high to allow robust inference of allelic ratios (data not shown).
Existing aberrations of the Ubx locus do not uncover a T2p leg enhancer
Regulatory mutations in Ubx cause morphological transformations similar to those characterized by clonal analysis with the Ubx null allele (Casanova et al., 1985; Kerridge and Morata, 1982; Peifer and Bender, 1986). We used this observation to attempt to map the regulatory regions of Ubx that control Ubx+ function in different regions of the legs. We examined all existing regulatory mutations of Ubx and new deficiencies that we generated by P-element-induced male recombination (Supp. Table 1). Fig. 3 illustrates the primary informative alleles. The results of this analysis are organized into three sections discussing first the regions 5′ of the Ubx transcript, the introns and then the regions 3′ of the Ubx transcript.
Fig. 3.
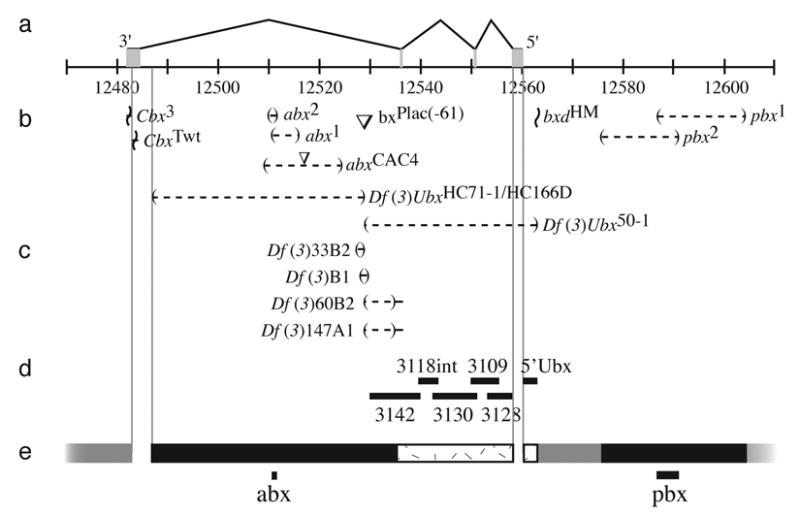
Map of the Ubx locus. (a) The four exons are illustrated above a scale showing genomic positions in kilobases (Drosophila melanogaster Genome Release 4.3). The centromere is to the left. (b) The most informative alleles used in this study are shown beside symbols indicating the type of lesion. Deletions are indicated by parentheses separated by a dashed line, insertions by inverted triangles (not to scale), inversion breakpoints by horizontal lines bisected by a wavy line. Uncertainty in the location of breakpoints is indicated by the range of the solid horizontal lines. (c) Four new deficiencies generated in this study by P-element induced male recombination using the bxPlac(−61) insertion are shown. (d) The positions of the six enhancer constructs are indicated with horizontal bars. (e) Regions scanned with deficiencies are indicated by black bars. Regions scanned by inversion are indicated by grey bars. Regions scanned with enhancer constructs are indicated with stippled boxes. The two minimal regions identified by this scan, the previously identified abx and pbx regions, are indicated with solid lines at the bottom of the figure. Regions of the Ubx gene not scanned by any technique are bounded by light lines extending from the molecular map vertically to the bottom of the figure.
The postbithorax region is required for Ubx expression in T3p: postbithorax (pbx) mutations cause transformations of T3p to T2p. The two known pbx mutations are deletions that overlap by approximately 5kb in the 5′ upstream regulatory region of Ubx (Fig. 3). As Lawrence (1979) reported, pbx homozygotes and hemizygotes displayed complete loss of the transverse rows on the T3 basitarsus and this is consistent with the loss of most Ubx expression in the posterior compartment of T3 leg imaginal discs (data not shown). The effects of pbx mutations on the T3p femur are more complicated.
In pbx hemizygotes and homozygotes, an apparently ectopic row of bristles appears on the lateral posterior femur of T3 together with a sharp column of trichomes (Fig. 4b). These legs carry seven bristle rows, which is equal to the wild-type number of rows. In contrast, large Ubx− clones lead to the derepression of an eighth bristle row (see above). Therefore, pbx alleles retain the ability to repress an eighth, lateral row of bristles on the posterior femur. The apparently ectopic row of bristles in pbx flies lies in the dorsal-most position of the posterior femur (see below). The bristles in this row probably represent the small bristles in the normal dorsal row that have been transformed to a more robust shape by loss of Ubx+ function. Finally, pbx mutations lead to the loss of the small bristles normally found on proximal T3p (cf. Figs. 1d with 4b). pbx T3p femurs still express low levels of Ubx at 24 hours APF, similar to the pattern observed in T2p (data not shown), suggesting that high levels of Ubx expression are required to specify these small proximal bristles, whereas low levels are sufficient to repress the eighth bristle row.
Fig. 4.
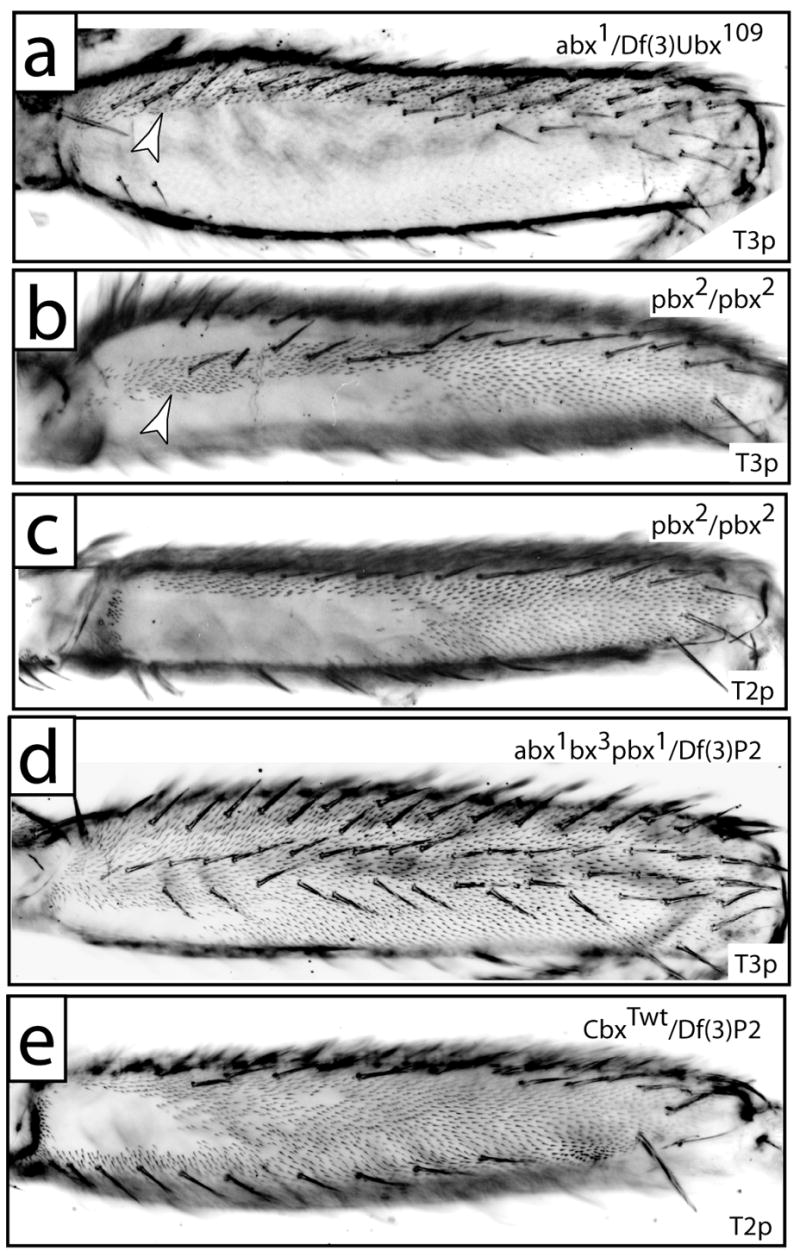
The posterior femurs of Ubx regulatory mutants. (a) T3p from a fly carrying abx1/Df(3)Ubx109 displays ectopic trichomes in the dorsal anterior compartment (arrowhead, compare with Fig. 2c). (b) T3p of a pbx2 homozygote displays a stripe of ectopic trichomes proximally (arrowhead) and distally. Naked cuticle is observed dorsally and ventrally to the ectopic proximal trichomes. (c) The distribution of naked cuticle on T2p of a pbx2 homozygote is approximately equal to the ventral naked cuticle on T3p (b). (d) T3p of a fly carrying abx1bx3pbx1/Df(3)P2 displays a trichome pattern that is approximately a composite of the abx and pbx patterns, with a naked patch of cuticle only on the posterior proximal surface. (e) T2p from a fly carrying CbxTwt/Df(3)P2 displays a wild type distribution of trichomes.
Four pieces of evidence indicate that the dorsal boundary between trichomes and naked cuticle in pbx mutants and Minute+ Ubx− clones reflects the anterior-posterior compartment boundary. First, wild type legs carrying en-lacZ demonstrate that cells in the dorsal anterior compartment of the proximal T3 femur differentiate naked cuticle (Fig. 5a). Second, the distribution of Ubx protein in the developing T3 legs of pbx hemizygotes reveals only one sharp boundary of Ubx expression that is coincident with the anterior-posterior compartment boundary, as revealed by marking the anterior compartment with anti-Ci staining, in both third-instar larvae and in 24 hour APF pupae (data not shown). Third, flies hemizygous for pbx and simultaneously carrying en-lacZ show a sharp boundary of en-lacZ staining coincident with the dorsal boundary of trichomes and naked cuticle (Fig. 5b). Fourth, ectopic expression of Ubx in T2 leads to repression of trichomes in the dorsal anterior region of T2 (Fig. 5c,d), suggesting that this dorsal anterior region is normally competent in both T2 and T3 to repress trichomes in response to high levels of Ubx expression. Together these observations support the hypothesis that in wild type flies, the naked cuticle on the proximal dorsal third femur extends into the anterior compartment, as shown in Fig. 1.
The effect of all bithoraxoid (bxd) mutations, large inversions that break between the pbx deletions and the first exon of Ubx, are identical to the effects observed with the pbx deletions (data not shown). This includes the allele bxdHM, which breaks close to the 5′ end of the Ubx transcript. Therefore, the enhancer(s) controlling expression in T2p are not found upstream of the bxdHM breakpoint.
The anterobithorax region is required for Ubx expression in T3a and Ubx function during early development in T2p and T3p
We examined all existing mutations that disrupt the large third intron of Ubx. All effects on the femurs resemble the effects of the anterobithorax (abx) mutations (Fig. 4a), which implies that the region defined by the small deficiency abx2 is required for Ubx expression in the anterior of the third femur. Patterns of Ubx protein expression in the imaginal discs of late-third instar larvae are consistent with this conclusion (data not shown).
abx mutations tested as homozygotes or in trans to deficiencies for the Ubx locus had no effect on T3p and derepressed proximal dorsal T3a trichomes (arrowhead in Fig. 4a), consistent with the effects of Ubx− clones in T3a (arrowhead in Fig. 2b). With rare exceptions (discussed below), abx mutations had no detectable effect on T2p trichome patterning (data not shown). Thus abx is not required for the Ubx expression that represses T2p trichomes during pupal development. Simultaneous removal of both the abx and pbx enhancers transformed T3p into the likeness of T2p, with derepression of distal trichomes, but maintenance of a naked proximal patch on both T2p and T3p (Fig. 4d). This result suggests that the regulatory element or elements that drive Ubx expression in the proximal patch of pupal legs are normally active in both T2p and T3p, but that their activity in T3p is normally hidden below the high levels of Ubx expression in T3p driven by the pbx enhancer.
Alleles of abx also generated a low frequency of posterior T2 femurs transformed towards a posterior T1 pattern, the postprothorax (ppx) transformation, similar to the penetrance observed by others (Casanova et al., 1985; Peifer and Bender, 1986). Rarely do ppx transformations completely transform the leg into a T1-like morphology. In cases of complete transformation, however, the transformation towards T1 is compelling, since the pattern of bristles resembles T1 and the legs show complete loss of naked cuticle in the proximal posterior of T2 (data not shown). This pattern of bristles and trichomes is indistinguishable from that obtained with Ubx− clones generated at 4±2 hours AEL (data not shown). In contrast, late Minute+ Ubx− clones show a small region of naked cuticle ventrally and a bristle pattern unlike T1 (Fig. 2c). Most ppx-transformed legs caused by abx contain small regions displaying a T1 transformation that resemble clonal patches. Combined with the observation that only Ubx− clones generated early in embryogenesis can cause the ppx transformation, these results suggest that the partial transformations result either from loss of Ubx+ function in a variable number of cells in the blastoderm, leading to derepression of Scr in these cells (Little et al., 1990; Struhl, 1982), or from derepression of Scr in only some Ubx− cells. The latter model is consistent with the observation that not all blastoderm clones show ppx transformation and that some clones show partial transformation (see Fig. 6 of Kerridge and Morata, 1982). We have also confirmed Little et al.’s (1990) report that ectopic Scr is observed at low frequency in patches in abx hemizygote T2 discs (not shown).
Fig. 6.
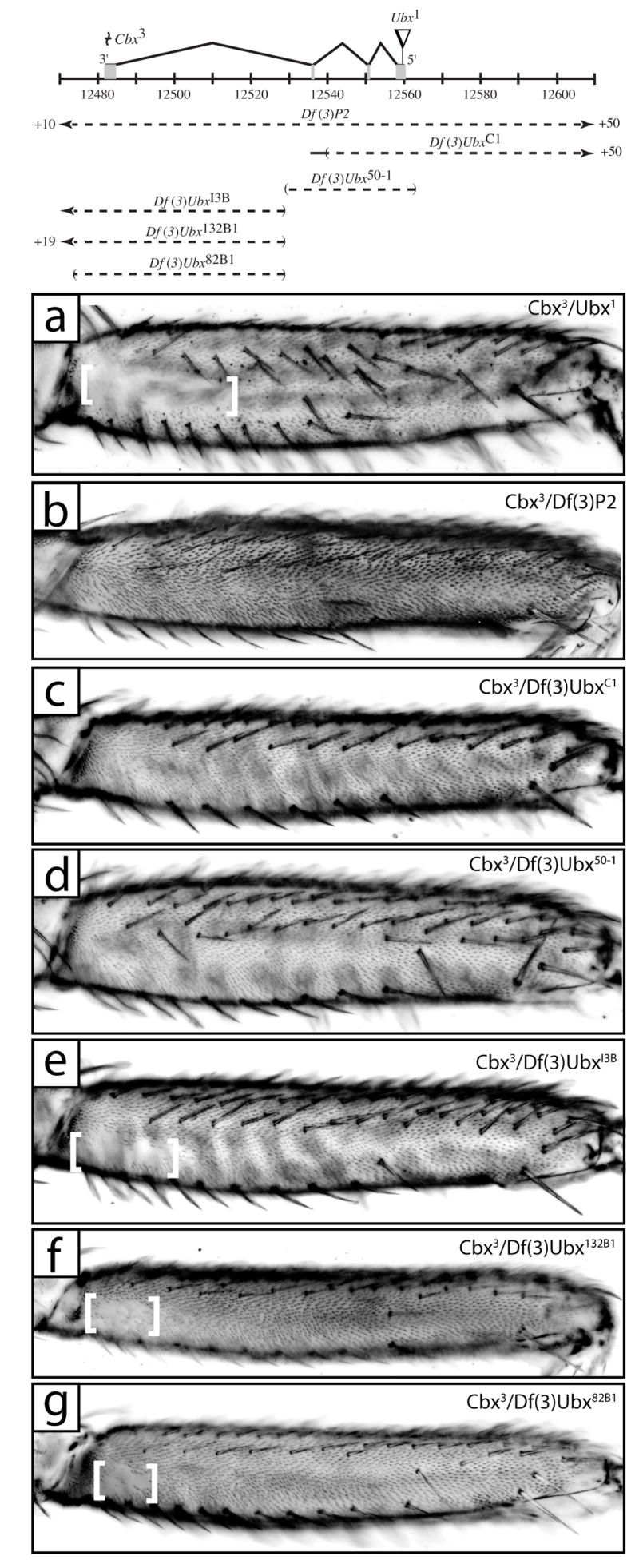
Cbx3 causes ectopic T2p and T3a trichomes in a transvection dependent manner. A map of the Ubx locus, as in Fig. 3, is shown at the top illustrating Cbx3 and Ubx1 lesions above and positions of deficiencies used in transvection mapping below. (a) T2p from a fly with the genotype Cbx3/Ubx1 displays a normal sized patch of naked cuticle. (b) T2p from Cbx3/Df(3)P2 flies exhibits only a small patch of proximal-ventral naked cuticle similar to that observed in Ubx clones (Fig. 2a). (c,d) T2p from flies with the genotype Cbx3/Df(3)UbxC1 (c) and Cbx3/Df(3)Ubx50-1 (d). (e,f,g) T2p from flies with the genotype Cbx3/Df(3)UbxI3B (e), Cbx3/Df(3)Ubx132B1 (f), and Cbx3/Df(3)Ubx82B1 (g) show a patch of proximal naked cuticle (between white brackets) indicative of trichome repression due to Ubx expression.
These results indicate that the abx enhancer region is required early in development to repress Scr in the second posterior femur and is required late for expression of Ubx in the anterior third femur. We found no evidence that the abx region is required for expression of Ubx in the posterior second femur after embryogenesis.
DNA 3′ of the Ubx transcript does not contain a T2p enhancer, but the Cbx3 inversion represses T2p function in cis
We examined two alleles containing inversions with breaks near the 3′ end of the Ubx transcript: CbxTwt and Cbx3. These alleles were originally identified because they display a dominant transformation of wing tissue towards a haltere phenotype (Bender et al., 1983). This gain of function phenotype is due to ectopic expression of Ubx anterior to its normal expression domain in wing tissue (Gonzalez-Gaitan et al., 1990; White and Akam, 1985). CbxTwt breaks closer to the Ubx third exon than does Cbx3. We did not detect any effect of CbxTwt on patterning of bristles or trichomes on the second (Fig. 4e) or third femur. Therefore, there is unlikely to be a regulatory region downstream of the Ubx transcript that is required for patterning bristles and trichomes on the femurs.
We were therefore surprised to find that the Cbx3 allele when homozygous or hemizygous displays a strong loss of naked cuticle on the proximal posterior second femur (Fig. 6b). This allele did not cause appearance of an ectopic row of bristles, suggesting that this allele is not leading to a strong loss of Ubx function during larval development in the posterior second femur. In addition, since the CbxTwt allele does not show this loss of naked cuticle (Fig. 4e), and because we have already excluded most of the region upstream of the Ubx transcript, we infer that the Cbx3 allele causes this loss of Ubx function through dominant suppression of an enhancer found within the region encoding the Ubx transcript. This model is supported by the fact that while we observe this loss of function in Cbx3 homozygotes and when the Cbx3 allele is placed in trans to a deficiency for the entire locus (Fig. 6b), we do not observe this loss of function phenotype when the Cbx3 allele is placed in trans to Ubx null alleles caused by point mutations (Fig. 6a). One model to explain this result is that the Cbx3 allele causes dominant repression of the T2p enhancer region mainly or entirely on the same chromosome (i.e. in cis). The recovery of Ubx function observed when Cbx3 is placed in trans to null point mutations would result from transvection, or the activation of the Ubx promoter on the Cbx3 chromosome by the T2p enhancer on the chromosome carrying the Ubx null allele (Lewis, 1954).
This hypothesis suggests an additional way to search for the T2p enhancer. If Cbx3 represses the T2p enhancer only in cis, then we would expect to observe loss of naked cuticle when Cbx3 is placed in trans to chromosomes carrying a deficiency including the T2p enhancer. In contrast, we would expect to observe a wild-type pattern of naked cuticle when Cbx3 is placed in trans to a deficiency that does not include the T2p enhancer. We have, in fact, observed loss of the T2p naked cuticle when Cbx3 is placed in trans to independent deficiencies that remove the first two introns and part of the third intron, but not when placed in trans to deficiencies that do not remove this region (Fig. 6c-g). These results support the hypothesis that the T2p enhancer is located within a region defined by the left-hand break of Df(3)UbxC1 and the right-hand break of Df(3)Ubx50-1 (Fig. 6), a region including mainly the first two introns of Ubx.
New deficiencies generated by P-element-induced male recombination fail to uncover an enhancer active in T2p
In order to generate additional deficiencies that might reveal the T2p enhancer, we used P-element-induced male recombination (Preston and Engels, 1996; Preston et al., 1996), using the rosy-containing P-element insertion plac(−61) located at the 3′ end of the large candidacy region, within the third intron of Ubx. We screened ~6,000 progeny and isolated 63 recombinants of which at least 45 were unique (0.75%). Of these 45, only 5 retained the P-element. At least 77% of our recombinants were associated with precise excisions of the P-element. This result is at odds with a previous study that reported that most male recombination events at cytological position 50C retained the P-element at its original site (Preston and Engels, 1996).
Of the five lines retaining the P-element, two were duplications and three were deficiencies in directions consistent with the Hybrid P-element Insertion model (Preston et al., 1996). Unfortunately, all three deficiencies were in the 3′ direction. Two (132B1 amd 82B1) were Ubx null with left-hand breakpoints extending beyond the Ubx last exon (Fig. 6). One (33B2) was a small deficiency of ~235bp (Fig. 3). Of the 3 lines that underwent imprecise excision of the P-element, one is a 200bp deficiency that gives a bx phenotype (B.1) and two are 5′ deficiencies of ~5–7kb (60B2 and 147A1) (Fig. 3). None of the non-Ubx− deficiencies have any detectable effect on the T2p trichome pattern either as homozygotes or when heterozygous with a deficiency removing the entire Ubx locus.
Enhancer constructs containing most of the remaining candidate regions of Ubx fail to drive expression in the pupal legs
None of the alleles discussed above definitively revealed a region controlling trichome patterning on T2p. These results rule out large regions 5′, 3′ and within the large last intron as containing cis-regulatory elements required for expression in T2p. This leaves two candidate regions.
The first is a small region between the left-hand break of Df(3)UbxHC71-1/HC166D and the right-hand break of CbxTwt, which includes ~2.8kb of the third intron 5′ of the last exon (Fig 3). We have not explored this 3′ region because none of our functional assays provide any support for the hypothesis that this region contains the leg enhancer. For example, none of the bx insertions, which presumably disrupt abx activity by introducing insulators between abx and the Ubx promoter (Peifer and Bender, 1986), disrupt the T2p trichome pattern. This suggests that the T2p enhancer is unlikely to be 3′ of the bx insertions.
The second region contains DNA between the bxdHM break and the right-hand break of Df(3)60B2 or Df(3)147A1, which contains ~3.2kb 5′ of the first exon, the first two introns and a portion of the third intron. We focused on this region because the transvection tests with the Cbx3 allele suggested that the T2p enhancer falls in this region.
We generated six enhancer constructs spanning 31kb, covering the entire large candidacy region except for the first exon (Fig. 3). As reported previously, the construct covering ~3.3kb 5′ of the transcription start site, drives expression in the visceral mesoderm in parasegment 7, part of the wild-type Ubx pattern, as well as ectopic expression in the gastric caecae in parasegment 4 (Bienz et al., 1988; Hursh et al., 1993; Irvine et al., 1991; Müller et al., 1989). Other than this pattern, we did not observe any expression patterns resembling wild-type expression during the embryonic, larval or pupal stages for any of the constructs.
DISCUSSION
One major challenge facing the field of evolutionary developmental biology is the identification of the individual nucleotide changes responsible for developmental and morphological evolution. This goal has rarely been achieved. This is largely because most evolutionary changes that have been identified are evolved expression patterns and we currently have a poor understanding of enhancer structure and evolution. We do not yet have reliable methods of predicting the location of enhancer regions and their identification typically requires brute-force empirical methods.
The particular evolutionary change we have studied, a change in the distribution of trichomes caused by an apparently slight shift in the quantity or distribution of Ubx protein, presents several challenges. First, one could explain our previous results with two competing hypotheses. The evolutionary change could have resulted from evolution of an enhancer that drives expression specifically in the pupal legs. Alternatively, the same results could be obtained through a change in the global (that is, non-tissue specific) cis-regulation of Ubx expression levels at the time when trichomes are repressed in T2p. In this case, the observed morphological change would have resulted from the fact that the extent of trichome repression on T2p is more sensitive to altered Ubx levels than other morphological features. We therefore tested whether the global levels of Ubx expression driven by the Ubx promoter have evolved between D. melanogaster and D. simulans. We used a method that measures relative expression levels between species due to changes in cis while controlling for differences in trans (Wittkopp et al., 2004). The relative levels of D. melanogaster and D. simulans Ubx mRNA were measured in D. melanogaster/D. simulans hybrid whole pupae. This is effectively a test for any global cis-regulatory difference that manifests as a difference in mRNA levels, including differences in the basal or other non-tissue specific promoters of Ubx, in portions of Ubx affecting transcript stability, or in the cis-targets of translational regulation mechanisms that result in mRNA degradation. We found no significant differences in the level of Ubx mRNA from the two species in hybrids. We conclude, therefore, that there is no evidence for evolved changes in the global cis-regulation of Ubx between D. melanogaster and D. simulans and that the most likely location of the evolutionarily relevant cis-regulatory change is in a leg-specific enhancer.
Our second challenge was thus to identify an enhancer driving Ubx expression in the pupal legs. We therefore embarked on a multi-pronged analysis to identify the regulatory regions controlling Ubx expression in T2p. We first performed a detailed analysis of the requirements for Ubx protein in the legs. This analysis confirmed that Ubx is required for repressing trichomes on the posterior second and third femurs. In addition, we discovered that Ubx is required to repress trichomes in a small region on the dorsal proximal anterior T3 femur and that a small region on the ventral posterior T2 does not require Ubx expression for repression of trichomes. Finally, we found that Ubx is required to repress an eighth row of bristles on the posterior second and third femurs and is required for the presence of the fine proximal bristles on T3p and to instruct the morphology and migration of the thin proximo-dorsal bristles on T3. When levels of Ubx expression are reduced in T3p by mutations of the pbx enhancer, some Ubx+ function remains that resembles the activity present in T2p. This weak expression, which can be observed by staining with anti-Ubx antibody (data not shown), accounts for the repression of trichomes in the proximal posterior femurs and the repression of the eighth bristle row on the posterior femur. This analysis is summarized in a model shown in Fig. 7 illustrating the requirements for expression driven by the abx and pbx regulatory regions and by unidentified enhancers required for a weak gradient of expression late in development on the posterior femurs of T2 and T3. The model emphasizes that both spatial and temporal differences in Ubx expression distinguish the function of Ubx in T2 and T3 (Stern, 1998).
Fig. 7.
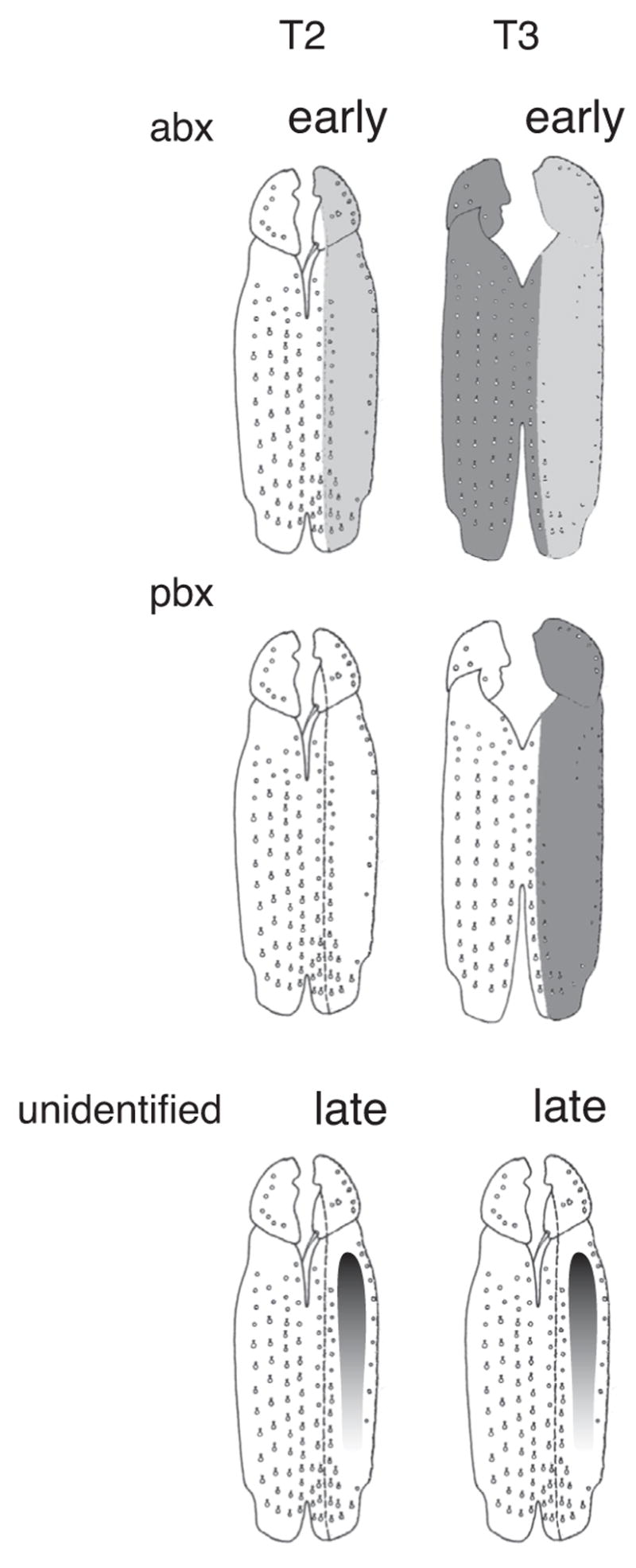
Domains of Ubx regulatory function in the femurs of Drosophila melanogaster. Weak expression is illustrated as light gray shading, strong expression as dark gray shading. The abx region contains elements promoting early embryonic expression in T2p and T3p and later high levels of expression in T3a. The pbx region contains elements required for T3p throughout development. Unidentified regions contain elements driving a proximal-distal expression gradient in T2p and T3p femurs.
We then attempted to identify the T2p regulatory region by an analysis of all existing alleles of the Ubx locus. This analysis confirmed that the pbx region is required for Ubx expression in the posterior third femur and that the abx region is required for expression in the anterior third femur and for expression in the blastoderm cells that will give rise to the posterior second femur. We generated several additional deficiencies in the large intron that also did not have any effect on leg patterning. We found no regulatory mutations that altered trichome patterning on T2p with the exception of Cbx3. This allele is an inversion with a breakpoint 3′ of the last exon of Ubx. We do not believe this allele removes a T2p enhancer because a different inversion allele with a breakpoint closer to the last Ubx exon (CbxTwt) does not alter trichome patterning. Instead, we infer that the Cbx3 allele causes repression of a T2p enhancer found elsewhere in the Ubx locus and that this repression is most effective on the enhancer in cis. One observation in support of this conclusion is that null alleles caused by small lesions placed in trans to Cbx3 drive Ubx expression in the correct T2p pattern from the Cbx3 allele. This is an example of transvection, or activation of a promoter by a cis-regulatory region in trans. This inference provided a potential method for narrowing down the region containing the T2p enhancer. We placed the Cbx3 allele in trans to a series of deficiencies that removed different parts of the Ubx locus. When the Cbx3 allele was placed in trans to alleles that removed the first two introns, the legs showed complete or nearly complete loss of naked cuticle. In contrast, when the Cbx3 allele was placed in trans to deficiencies that removed mainly the third intron, the naked cuticle looked wild type. This experiment suggested that the T2p enhancer is located between the limits defined by the left-hand breakpoint of Df(3)UbxC1 and the right-hand breakpoint of Df(3)Ubx50-1.
We then assayed almost the entire region defined by these breakpoints using enhancer constructs. We found that none of these constructs possessed enhancers that, on their own, are capable of driving expression of a reporter gene in the posterior epidermis of the second femur. There are several possible explanations for our failure to find the T2p enhancer. One trivial explanation is that the enhancer lies in one of the several small regions we have not yet surveyed, including the first and last exons and a 2.8kb region 5′ of the latter. There is little possibility that enhancer elements required for T2p expression exist upstream or downstream of the Ubx transcript or in the portion of the third exon that we were able to test with deficiencies (Fig. 3). Another possibility is that the enhancer for T2p does exist in the area of the first two introns, but is complex such that the transcription factor binding sites that drive this expression are scattered across a region larger than any of our individual enhancer constructs (Klingler et al., 1996). A final possibility is that the enhancer for T2p is dependent for its function on its presence within the Ubx locus and cannot be identified by removal from this context.
Supplementary Material
Acknowledgments
We thank Michael Akam, Marion Rozowski, Henrique Teotonio and several anonymous referees for helpful comments on earlier drafts of this manuscript. Genomic clones were kindly provided by Michael Akam. Fly stocks were kindly provided by Ed Lewis, Michael Akam, Antonio Garcia-Bellido, Welcome Bender, and the Bloomington and Umeå stock centers. We thank Rita Pasini for Drosophila injections of construct 3118int, Rob White for providing the FP3.38 antibody, and Robert Holmgren for the anti-Cubitus interruptus antibody. We thank Andrew Clark for use of Pyrosequencing equipment and supplies. This research was supported by a NRSA Fellowship to GKD (GM69102), a Damon Runyon Cancer Research Postdoctoral Fellowship to PJW (DRG # 1795-03) and a Junior Research Fellowship from Churchill College, Cambridge, a David Phillips Research Fellowship from the Biotechnology and Biological Sciences Research Council of the U.K., a grant from The National Institutes of Health (GM063622) and a David & Lucile Packard Foundation Fellowship to DLS.
Footnotes
Individual responsible for examining proofs, etc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. [Google Scholar]
- Averof M, Patel NH. Crustacean appendage evolution associated with changes in Hox gene expression. Nature. 1997;388:682–686. doi: 10.1038/41786. [DOI] [PubMed] [Google Scholar]
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques 29, 726, 728. 2000;730:732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- Bender W, Akam M, Karch F, Beachy PA, Peifer M, Spierer P, Lewis EB, Hogness DS. Molecular genetics of the bithorax complex in Drosophila melanogaster. Science. 1983;221:23–29. doi: 10.1126/science.221.4605.23. [DOI] [PubMed] [Google Scholar]
- Bienz M, Saari G, Tremml G, Müller J, Züst B, Lawrence PA. Differential regulation of Ultrabithorax in two germ layers of Drosophila. Cell. 1988;53:567–576. doi: 10.1016/0092-8674(88)90573-9. [DOI] [PubMed] [Google Scholar]
- Casanova J, Sánchez-Herrero E, Morata G. Prothoracic transformation and functional structure of the Ultrabithorax gene of Drosophila. Cell. 1985;42:663–669. doi: 10.1016/0092-8674(85)90123-0. [DOI] [PubMed] [Google Scholar]
- Gompel N, Prud’Homme B, Wittkopp PJ, Kassner VA, Carroll SB. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature. 2005;433:481–487. doi: 10.1038/nature03235. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan MA, Micol JL, Garcia-Bellido A. Developmental genetic analysis of Contrabithorax mutations in Drosophila melanogaster. Genetics. 1990;126:139–155. doi: 10.1093/genetics/126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah-Alava A. Morphology and chaetotaxy of the legs of Drosophila melanogaster. Journal of Morphology. 1958;103:281–310. [Google Scholar]
- Hursh DA, Padgett RW, Gelbart WM. Cross regulation of decapentaplegic and Ultrabithorax transcription in the embryonic visceral mesoderm of Drosophila. Development. 1993;117:1211–22. doi: 10.1242/dev.117.4.1211. [DOI] [PubMed] [Google Scholar]
- Irvine KD, Helfand SL, Hogness DS. The large upstream control region of the Drosophila homeotic gene Ultrabithorax. Development. 1991;111:407–424. doi: 10.1242/dev.111.2.407. [DOI] [PubMed] [Google Scholar]
- Kerridge S, Morata G. Developmental effects of some newly induced Ultrabithorax alleles of Drosophila. Embyrol exp Morph. 1982;68:211–234. [PubMed] [Google Scholar]
- Klingler M, Soong J, Butler B, Gergen JP. Disperse versus compact elements for the regulation of runt stripes in Drosophila. Dev Biol. 1996;177:73–84. doi: 10.1006/dbio.1996.0146. [DOI] [PubMed] [Google Scholar]
- Landry CR, Wittkopp PJ, Taubes CH, Ranz JM, Clark AG, Hartl DL. Compensatory cis-trans evolution and the dysregulation of gene expression in interspecific hybrids of Drosophila. Genetics. 2005;171:1813–22. doi: 10.1534/genetics.105.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Morata G. Bristle patterns and compartment boundaries in the tarsi of Drosophila. J Embryol exp Morph. 1979;51:195–208. [PubMed] [Google Scholar]
- Lewis EB. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. American Naturalist. 1954;88:225–239. [Google Scholar]
- Little JW, Byrd CA, Brower DL. Effect of abx, bx and pbx mutations on expression of homeotic genes in Drosophila larvae. Genetics. 1990;124:899–908. doi: 10.1093/genetics/124.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfooz NS, Li H, Popadic A. Differential expression patterns of the hox gene are associated with differential growth of insect hind legs. Proc Natl Acad Sci U S A. 2004;101:4877–82. doi: 10.1073/pnas.0401216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RS, Hogness DS. Functional dissection of Ultrabithorax proteins in D. melanogaster. Cell. 1990;60:597–610. doi: 10.1016/0092-8674(90)90663-y. [DOI] [PubMed] [Google Scholar]
- McCall K, O’Connor MB, Bender W. Enhancer traps in the Drosophila bithorax complex mark parasegmental domains. Genetics. 1994;138:387–399. doi: 10.1093/genetics/138.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, Garcia-Bellido A. Developmental analysis of some mutants of the bithorax system of Drosophila. Wilhelms Roux’s. Archives of Developmental Biology. 1976;179:125–143. doi: 10.1007/BF00848298. [DOI] [PubMed] [Google Scholar]
- Morata G, Kerridge S. Sequential functions of the bithorax complex of Drosophila. Nature. 1981;290:778–781. doi: 10.1038/290778a0. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Developmental Biology. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Müller J, Thuringer F, Biggin M, Züst B, Bienz M. Coordinate action of a proximal homeoprotein binding site and a distal sequence confers the Ultrabithorax expression pattern in the visceral mesoderm. EMBO J. 1989;8:4143–4151. doi: 10.1002/j.1460-2075.1989.tb08599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Bender W. The anterobithorax and bithorax mutations of the bithorax complex. The EMBO Journal. 1986;5:2293–2303. doi: 10.1002/j.1460-2075.1986.tb04497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CR, Engels WR. P-element-induced male recombination and gene conversion in Drosophila. Genetics. 1996;144:1611–22. doi: 10.1093/genetics/144.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CR, Sved JA, Engels WR. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics. 1996;144:1623–38. doi: 10.1093/genetics/144.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y, Cheung U, Larsen EW, Eberl DF. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in drosophila. Genesis. 2002;34:115–8. doi: 10.1002/gene.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL. A role of Ultrabithorax in morphological differences between Drosophila species. Nature. 1998;396:463–466. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL, Sucena E. Preparing larval and adult cuticles for light microscopy. In: Ashburner M, Hawley S, Sullivan B, editors. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2000. pp. 601–615. [Google Scholar]
- Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proceedings of the National Academy of Sciences, USA. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chamberlin HM. Evolutionary innovation of the excretory system in Caenorhabditis elegans. Nat Genet. 2004;36:231–2. doi: 10.1038/ng1301. [DOI] [PubMed] [Google Scholar]
- White RAH, Akam ME. Contrabithorax mutations cause inappropriate expression of Ultrabithorax products in Drosophila. Nature. 1985;318:567–569. [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. Evolutionary changes in cis and trans gene regulation. Nature. 2004;430:85–8. doi: 10.1038/nature02698. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. Parent-of-origin effects on mRNA expression in Drosophila melanogaster not caused by genomic imprinting. Genetics. 2006;173:1817–21. doi: 10.1534/genetics.105.054684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


