Summary
The activation of NMDA receptors (NMDARs) triggers long-term changes in AMPA receptor-mediated synaptic transmission in the CNS. These long-lasting changes occur via the addition or removal of AMPA receptors (AMPARs) at the synaptic membrane and are mediated by a number of regulatory proteins including the GluR2 AMPAR-interacting proteins n-ethylmaleimide sensitive factor (NSF) and Protein Interacting with C Kinase (PICK1). We have shown that the potent activation of NMDARs drives unclustering of PICK1 and PICK1-GluR2 dissociation in dendrites resulting in increased surface delivery of AMPARs. Here we show that the dispersal of PICK1 is mediated by the actions of NSF. We find that elevated NMDAR signaling leads to the S-nitrosylation of NSF and increased NSF-GluR2 association. Both NMDAR-dependent unclustering of PICK1 and the delivery of surface AMPARs is dependent on release of nitric oxide (NO). Our data suggest that NMDAR activation can drive the surface delivery of AMPARs from a pool of intracellular AMPARs retained by PICK1 through the NO-dependent modification of NSF.
Keywords: NMDA, AMPAR trafficking, S-nitrosylation, PICK1, NSF
Introduction
NMDA receptor-induced increases in AMPAR trafficking to the synaptic surface mediates changes in synaptic efficacy in a number of brain regions (Shi et al. 1999; Sun et al. 2005; Frenkel et al. 2006). While this process has been linked to the regulated insertion of GluR1-containing AMPARs, subsequent insertion of GluR2-containing receptors are also critical to maintaining synaptic potentiation (Hayashi et al. 2000; Passafaro et al. 2001; Plant et al. 2006). However, evidence for a role or mechanism of regulated GluR2-containing AMPARs insertion is limited. We have previously found that elevated NMDAR activation unclusters dendritic PICK1 and resulted in the release of GluR2-containing AMPARs (GluR2-AMPARs) to the membrane surface (Sossa et al. 2006). This PICK1-dependent increase in surface AMPARs suggests that GluR2 localization with and retention by PICK1 in intracellular pools serves as a site of regulation for receptor-induced changes in synaptic transmission. To provide further insight into the mechanisms of such NMDAR-induced plasticity, further examination of the mechanisms that regulate PICK1 clustering is required.
The process of modulating GluR2-AMPAR levels at synapses is regulated by a number of AMPA receptor interacting proteins (Braithwaite et al. 2000; Kim et al. 2004), one of which is N-ethylmaleimide sensitive factor (NSF). NSF chaperones GluR2-AMPARs to the membrane surface and disassembles PICK1 from GluR2-AMPARs (Hanley et al. 2002), perhaps freeing pools of intracellularly anchored AMPARs to return to the membrane surface. Activation of NSF by nitric oxide (NO)-dependent S-nitrosylation increases the binding of NSF to GluR2 and enhances the surface expression of AMPARs (Huang et al. 2005). We have, therefore, investigated whether the PICK1-dependent delivery of AMPARs following NMDAR activation is mediated by the NSF and its modification by NO.
We find that dendritic PICK1 unclustering and the subsequent delivery of AMPARs to the membrane surface occurs via NMDAR-mediated S-nitrosylation of NSF. NMDAR stimulation increases NO production and S-nitrosylation of NSF. Consistent with the possibility that S-nitrosylation increases NSF function, we find that NMDA/low Mg2+ treatment increases NSF-GluR2 association and surface AMPAR levels. NO donors mimic the NMDA/low Mg2+-induced decrease in dendritic PICK1 levels, a result blocked by NO scavengers. We show that the NMDA/low Mg2+-induced loss in PICK1 labeling is an NO-dependent process, which leads to enhanced NSF function and subsequent increase in surface AMPAR expression.
Material and Methods
Reagents and antibodies
Antibodies
PICK1 (Affinity Bioreagents; rabbit polyclonal 1:1000 for IF), GluR2 (Chemicon International, Inc.; Temecula, CA; N-terminal mouse monoclonal 5 μg for IP and 1:100 for IF), NSF antibody (BD Transduction Laboratories, San Jose, CA; mouse monoclonal 1:1000 for Western blotting)
Primary hippocampal cultures
Cultured hippocampal neurons were prepared as previously described (Sossa et al. 2006). Hippocampi were dissected out of P0 rat pups, dissociated with papain, and plated on poly-D-lysine coated coverslips. For the first 36 hours cells fed on MEM media with fetal bovine serum. Then neurons were maintained on Neurobasal media (GIBCO, Invitrogen Corp, Grand Island, NY) with B27 (GIBCO) and glutamax (GIBCO). Experiments were performed using 14 to 21 DIV neurons.
Immunofluorescence and Quantitation
After fixation and primary antibody labeling, neurons were incubated with a secondary antibody conjugated with a fluorescent moiety. This allowed the signal to be visualized via fluorescence microscopy. Images were taken at 60X magnification using a Hamamatsu ORCA ER camera attached to a Nikon microscope. Signals from immunolabeled neurons were analyzed using Metamorph software (Universal Imaging, Inc., Downingtown, PA). Following background subtraction and thresholding, which included only signals 2-fold greater than the diffuse labeling in dendritic shafts, we obtained an integrated signal intensity value for the dendritic regions. These values were then normalized to the area of the dendritic region and graphed. We performed 4-5 experiments (n = 4-5) with 7-20 images of each condition taken, and where applicable the data is presented as a percent change from the appropriate control. Experiments were analyzed by an individual blinded to treatment conditions.
Neuron Treatments
NMDA receptor activation was achieved with 1 minute exposure to NMDA (20 μM; Tocris Cookson, Inc., Ellisville, MO, with 10 μM CNQX (Tocris), 1 μM MPEP (Tocris); 1 μM LY367385 (Tocris)) in low Mg2+ (0.13 mM) ACSF termed NMDA/low Mg2+). (Normal ACSF contained (in mM): 119 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 1 NaH2PO4, 1.3 MgSO4, and 2.5 CaCl2). After briefly washing cells with conditioned medium, they were returned to 37°C for 14 minutes. Follow this incubation time, cells were fixed and immunocytochemically processed for PICK1 or surface AMPARs. Neurons treated with SNAP (S-nitroso-N-acetylpenicillamine, Calbiochem, 100 μM for 10 min) or PTIO (Calbiochem, 50 μM for 5 min). We also used water soluble peptides designed to inhibit NSF-mediated delivery of AMPARs by blocking NSF-SNAP association (Lledo et al. 1998). We used cell permeable TAT-conjugated peptides to deliver NSF-SNAP inhibition peptides (AnaSpec Corp., San Jose, CA). The NSF peptide mimics the SNAP binding site for NSF (H-QSFFSGLFGGSSKIEEACE-GYGRKKRRQRRR-NH2); whereas the control peptide is a scrambled sequence (H-GFAESLFQSIEKESGFSCG-GYGRKKRRQRRR-NH2). Control or NSF peptide (100 μM; 15 minutes prior to NMDA/low Mg2+ treatment and during treatment and post-treatment phase as well; the neurons are exposed to peptides for a total of 30 min).
siRNA Experiments
Hippocampal cultures, 11-13DIV, were transfected with negative control siRNA or siRNAs against PICK1 using lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to a procedure specified by the manufacturer. PICK1 and control siRNA were purchased from Invitrogen. Following incubation for 2 hrs, the cells were washed in conditioned media and kept at 37°C. Neurons were treated with drugs as specified and immunocytochemically processed for labeling of PICK1 or surface GluR2 after 3 days.
GluR2 surface assay
Neurons were pretreated with SNAP, PTIO, NMDA/low Mg2+ or NMDA/low Mg2+/PTIO prior to live labeling using a GluR2 antibody (25 min 4°C). After labeling for AMPARs the coverslips were fixed with 4% PFA (15 min) and immunocytochemically processed for surface receptors using a fluorescently conjugated secondary antibody. As AMPARs are labeled with a mouse monoclonal GluR2 antibody, we used Cy3-conjugated donkey anti-mouse secondary.
NSF-GluR2 coIP
Slice preparations were made from 2-3 week old rat pups as described previously (Sossa et al. 2006). Following treatment with control ACSF or ACSF containing NMDA/low Mg2+ (10 μM, 1 min; plus cocktail of inhibitors described in neuronal treatments methods section), the 400 μm thick slices were homogenized using a Hepes-EDTA-Neocuproine (HEN) Buffer (250 mM (pH 7.7), 1 mM EDTA, 0.1 mM neocuproine; with protease inhibitor cocktail (Complete, Mini, EDTA-free, Roche Diagnostics, Mannheim, Germany) and 1mM ATPγS (Sigma-Aldrich)). Slices underwent 2 series of homogenization and centrifugation (5 min., 1000 rpm) steps before adding Triton TX-100 (1%; 20 min, 4°C). After another round of homogenization-centrifugation, the supernatant was collected and stored at -80°C until initiation of coimmunoprecipitation. Five hundred micrograms of protein and 5 μg of GluR2 antibody (Chemicon) were incubated overnight while rocking at 4°C. After washing protein G agarose beads in lysis buffer, the protein-antibody complex was added to the beads for 2 hours at 4°C with continuous rocking. Following boiling (5 min) of bead-antibody-protein complex in loading buffer (containing reducing agent 5% β-mercaptoethanol), samples were run on 10% SDS PAGE gel. Separated proteins were then transferred onto nitrocellulose membrane and subsequently blotted with NSF antibody. Band signals were detected using Amersham ECL™ Western Blotting detection reagents (GE Healthcare Bio-Sciences Corp.; Piscataway, NJ), which enable the chemifluorescence detection of proteins via horse radish peroxidase-conjugated secondary antibodies. We visualized signals from bands on BioMax luminescent film (Fisher Scientific). Bound intensities were normalized to input intensities and presented as a ratio.
NO levels assay
To determine if nitric oxide (NO) is produced under our conditions, we employed a NO assay kit (EMD Biosciences, Inc., San Diego, CA) that colormetrically measures the conversion of nitrate to nitrite by nitrate reductase. We quantified NO levels spectrophotometrically using Griess reagent at 540 nm.
Biotin Switch Method and detection of S-nitrosylated NSF
We purified nitrosylated proteins from slice homogenates using the Biotin Switch Method for detection of S-nitrosylated proteins (Jaffrey et al. 2001). Samples are run on 10% SDS PAGE gels and transferred to nitrocellulose membranes. Detection and analysis of NSF (1:1000) was performed as described in the coIP methods section.
Electrophysiology
Whole cell patch recordings were made from 2-3 week old cultured hippocampal neurons. Miniature EPSCs were recorded with a Multiclamp Amplifier (Molecular Devices, Sunnyvale, CA) using low-resistance electrodes (3-5 MΩ). The pipette solution contained: (in mM) 125 K gluconate, 10 NaCl, 10 HEPES, 10 EGTA, 5 Sucrose, 4 MgATP, 0.3 NaGTP pH 7.2. The extracellular solution contained 120 NaCl, 26 NaHCO3, 10 Glucose, 2,5 KCl, 1 NaH2PO4, 20 Glucose, 2.5 CaCl2, 1.3 MgSO4 and adjusted to pH 7.4. Extracellular solution was infused with 95% O2/5% CO2 and contained 100μM lidocaine. The stability of series and input resistances were confirmed throughout the experiment using Igor Pro Software (Wavemetrics, Lake Oswego, OR). mEPSCs were collected from control and parallel slips treated for 1 minute with NMDA/low Mg2+. Recordings were made 15 minutes after drug application. 10min of mEPSCs were collected per cell. For each n, several cells were recorded from a single control or NMDA treated coverslip which were otherwise maintained under identical conditions. mEPSCs were analyzed using the Mini Analysis Program (Synaptosoft, Decatur, GA).
Results
NMDA/low Mg2+-dependent unclustering of dendritic PICK1 requires NSF activity
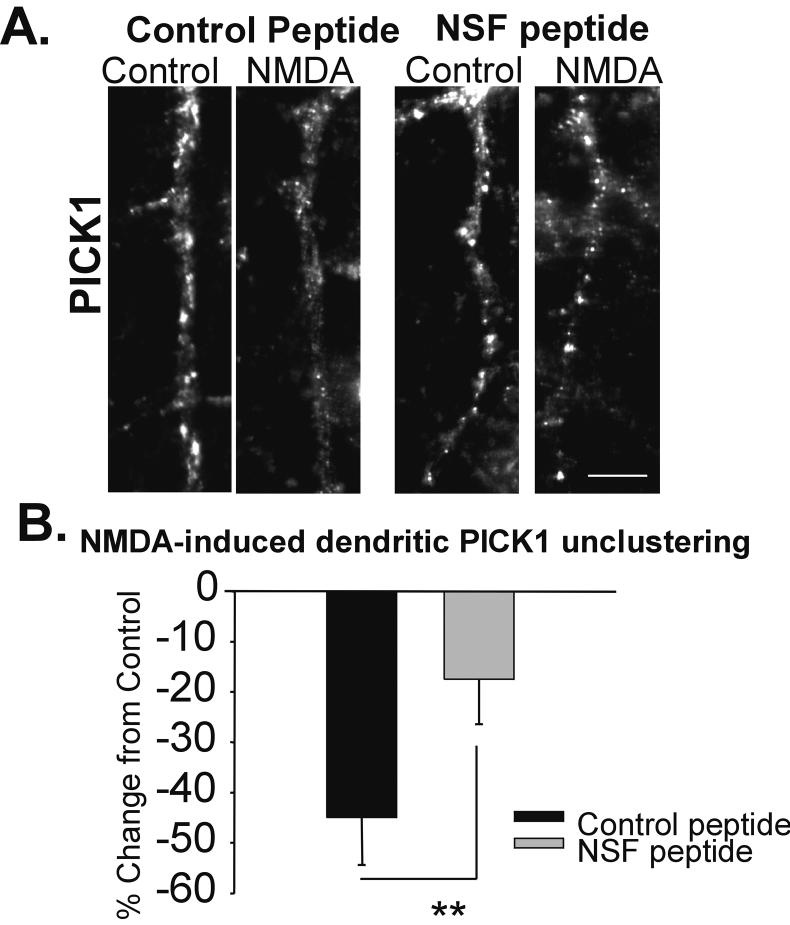
We have previously reported that brief, potent pharmacological activation of NMDARs (20 μM, 1 min in low Mg2+ ACSF) results in the unclustering of dendritic PICK1 (Sossa et al. 2006). The disassembly of PICK1 from the GluR2 AMPAR subunit has been shown to be enhanced by the ATPase activity of NSF when bound to Soluble NSF Associated Protein (Hanley et al. 2002), resulting in enhanced surface delivery of AMPARs. To investigate whether NMDA/low Mg2+ treatment may uncluster PICK1 through regulating the function of NSF, we used peptides that inhibit the interaction of NSF and the Soluble NSF Associated Protein, thus reducing the ATPase activity of NSF and blocking exocytosis. Control and NSF blocking peptides were designed with a TAT sequence, which enables the peptide to cross the membrane barrier (Lledo et al. 1998). Cells loaded with the control peptide exhibited a sharp decrease in the dendritic clustering of PICK1 following NMDAR activation in the presence of low Mg2+ (20 μM NMDA, 1 min, 0.13 mM Mg2+; NMDA-low Mg2+/Control -45 ± 9.4%; n= 4, Fig. 1A, B). In contrast, cells incubated with the NSF peptide showed significantly less unclustering following NMDA/low Mg2+ treatment (NSF peptide: NMDA-low Mg2+/Control -17.5 ± 9 %; n= 4; p< 0.01; Fig. 1A, B). NSF, and its regulated ATPase activity, therefore, are important for regulated PICK1 unclustering.
Figure 1. NMDA/low Mg2+-induced PICK1 unclustering is dependent on NSF.
A, Images of representative neuronal dendrites labeled for PICK1 in hippocampal cells were loaded with a TAT-linked Control (scrambled) or NSF disrupting peptide for 15 minutes prior to treatment with ACSF ± NMDA/low Mg2+ (NMDA). While control peptide treated neurons showed NMDA/low Mg2+-induced loss of dendritic PICK1, this decrease was greatly reduced in cells loaded with the NSF disrupting peptide. B, Quantitation of A (Scale bar = 5 μm; n= 4, ** p< 0.01)
Enhanced NMDAR activation is associated with increased NSF S-nitrosylation
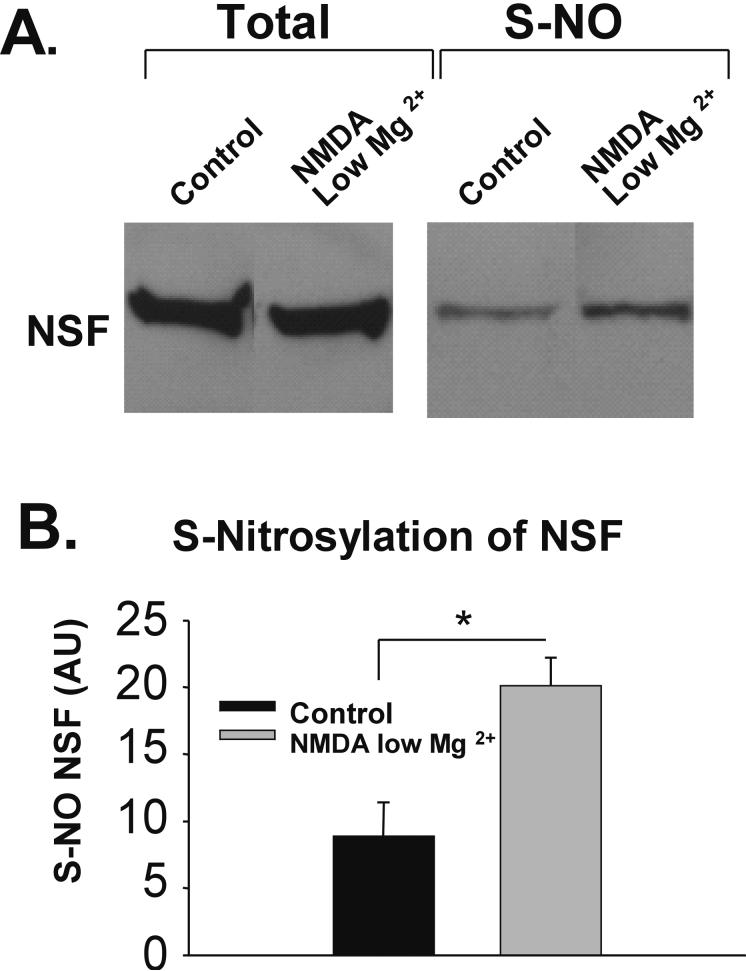
A possible mechanism by which NMDARs may be coupling to the function of NSF is through S-nitrosylation of NSF (Huang et al. 2005). To evaluate whether S-nitrosylation of NSF activity is increased by NMDA/low Mg2+ treatment, we prepared hippocampal slices (400 μm; from 14-21 PND rats) and treated them with ACSF or ACSF with NMDA/low Mg2+. We then isolated S-nitrosylated proteins (using the biotin switch technique; see (Jaffrey and Snyder 2001); which involves the substitution of S-nitrosyl moieties with biotin and culminates in a streptavidin pull-down of S-nitrosylated proteins. Western blotting for NSF revealed that NMDA/low Mg2+ resulted in greater S-nitrosylation of NSF compared to control (Ctrl 8.9 ± 2.5; NMDA/low Mg2+ 20.1 ± 2.1; n=3, p< 0.05; Fig. 2A, B). These results demonstrate that NMDA/low Mg2+ increases NSF S-nitrosylation.
Figure 2. NMDA/low Mg2+-treatment increases S-nitrosylation of NSF.
A, NSF in lysates from NMDA/low Mg2+-treated and untreated hippocampal slices, was assayed for levels of S-nitrosylation. After performing a biotin-switch protocol, S-nitrosylated proteins were isolated and probed for NSF by Western blot. While NSF levels are shown to be equal in total protein fractions, the S-nitrosylated lanes show that NMDA/low Mg2+ increased the amount of modified NSF. B, Quantitation of A. The unnormalized data is graphed in arbitrary units (AU). (n= 3; * p< 0.05)
Strong NMDAR activation causes increased NSF-GluR2 association
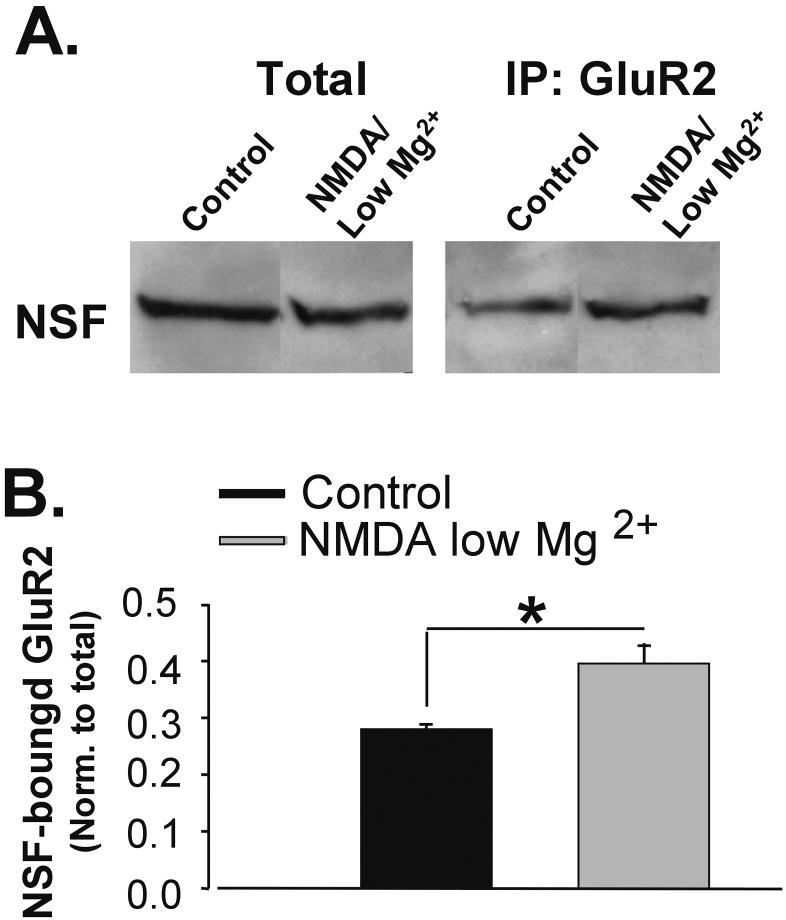
It has been reported that S-nitrosylation of NSF can increase the binding of NSF to GluR2-AMPARs (Huang et al. 2005). We therefore determined if NMDA/low Mg2+, which increases NSF activity, subsequently modifies NSF association with GluR2. We immunoprecipitated GluR2-AMPARs from hippocampal slices treated as described above. Western blots of immunoprecipitated proteins were probed for NSF. In NMDA/low Mg2+-treated lysates more NSF was coimmunoprecipitated with GluR2 (Ctl 0.28 ± 0.09, NMDA-low Mg2+ 0.39 ± 0.04; n= 5, p<0.05; Fig. 3A, B). Thus, NMDA/low Mg2+ treatment may enhance the association of NSF with GluR2 and may position NSF to alter PICK1 binding to GluR2.
Figure 3. NMDA/low Mg2+ increases GluR2-NSF association.
A, GluR2-NSF association was assayed by coimmunoprecipitation. Hippocampal slices were treated with ACSF ± NMDA/low Mg2+. GluR2 and associated proteins were isolated by immunoprecipitation. Western blotting for NSF protein revealed that NMDA/low Mg2+ treatment increased the amount of NSF associated with GluR2. B, Quantitation of A. Immunprecipitated NSF band intensities are normalized to total NSF protein levels (n=5, * p< 0.05).
NO donors uncluster PICK1 and increase surface GluR2 expression
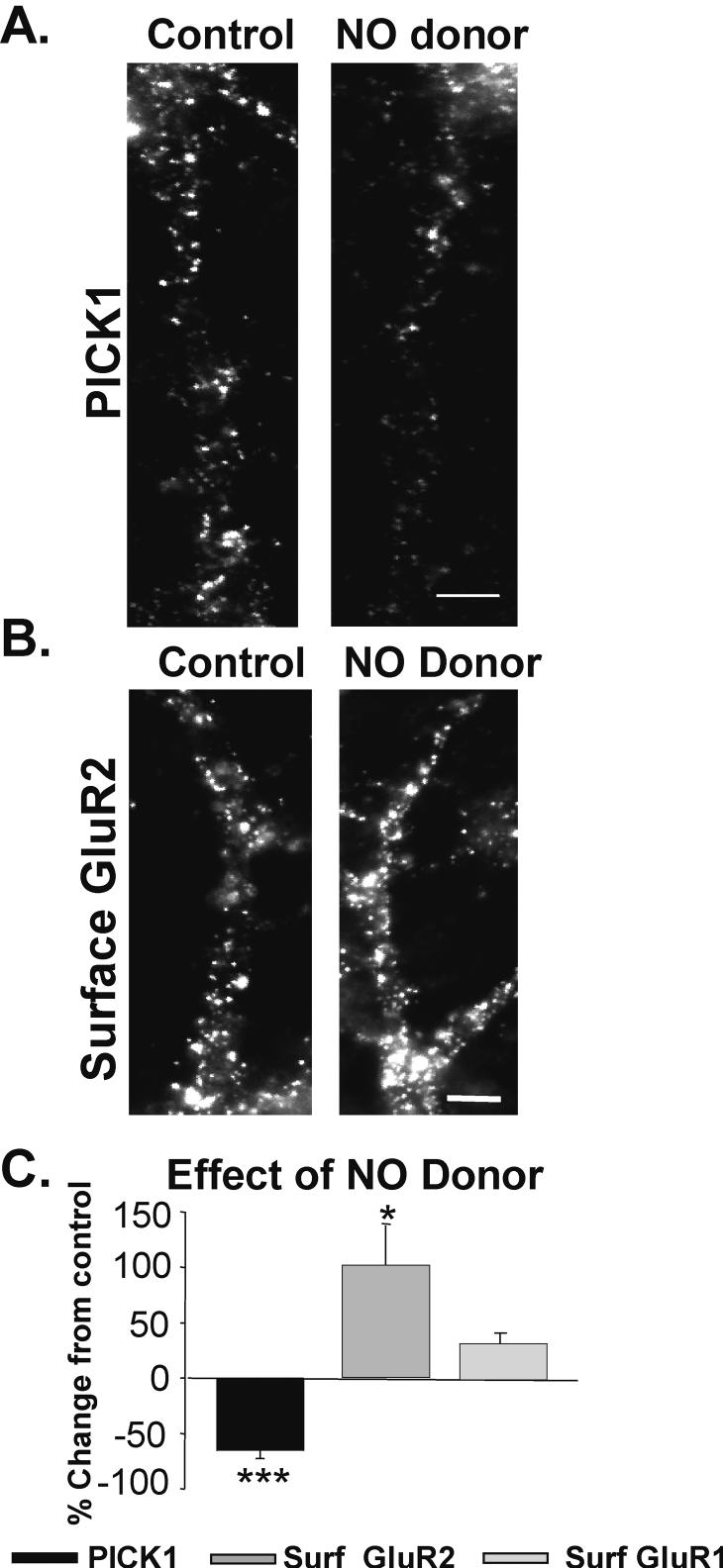
We next determined whether the S-nitrosylation of NSF, independent of NMDAR activation, could account for the unclustering of PICK1. S-nitrosylation of proteins, including NSF, is mediated by nitric oxide (NO). We, therefore, treated neuronal cultures with a NO donor (S-nitroso-N-acetylpenicillamine; SNAP; 100 μM, 10 min). Cells were then fixed and immunocytochemically processed for PICK1. We found that SNAP treatment alone caused PICK1 unclustering (SNAP/Ctrl -54.3 ± 7%; n= 7, p< 0.001; Fig. 4A, C-black bar).
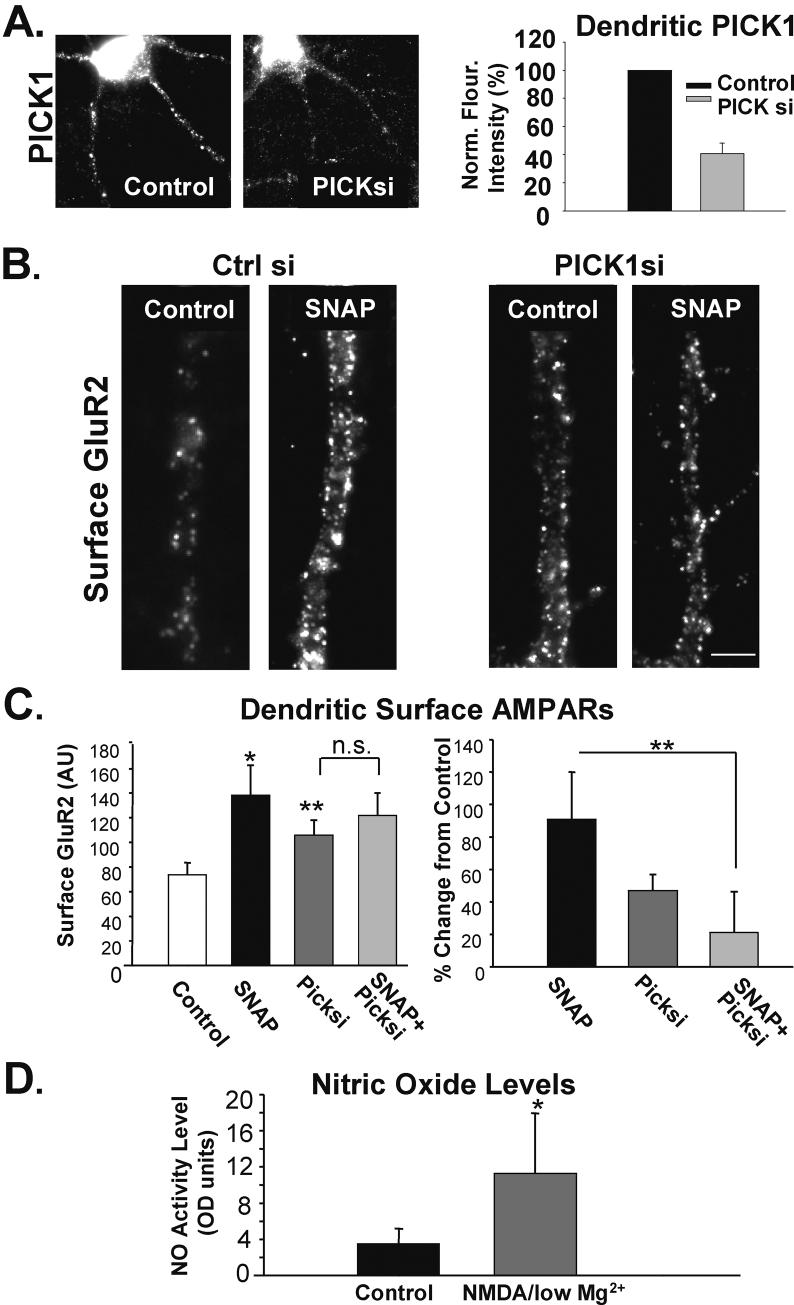
Figure 4. NO decreases dendritic PICK1 levels, while increasing surface AMPARs.
A, Images depict dendrites immunocytochemically labeled for PICK1 in neurons treated with and without NO donor (SNAP; 100 μM; 10 min, n = 7). B, Images of similarly treated cells labeled for surface GluR2-AMPARs. (n = 3, Scale bar = 3 μm) C, Quantitation of B and C reveals that NO reduces PICK1 in dendrites while enhancing the surface expression of GluR2 and GluR1 to a lesser extent (* p< 0.05, ***p< 0.001).
Previously, we showed that PICK1 unclustering following NMDAR activation resulted in higher surface AMPAR levels (Sossa et al. 2006). Consequently, we tested whether PICK1 unclustering by NO would similarly be associated with an increased expression of surface AMPARs. We treated neurons with SNAP (100 μM, 10 min) and subsequently labeled for surface AMPARs (30 min, 4°C). Consistent with our hypothesis, we found that SNAP-induced PICK1 unclustering was associated with an increase in surface GluR2 AMPAR expression (SNAP 102.3 ± 36.0% increase over control levels; n=5, p< 0.05; Fig. 4B, C-gray bar). However, analysis of changes in surface GluR1 levels demonstrated a much more modest increase accompanying SNAP treatment (SNAP/Ctrl 35.3 ± 11.3% increase over control levels; n=5, Fig 4C) suggesting this effect may exhibit AMPAR subunit specificity.
NO-mediated delivery of surface AMPARs is dependent on PICK1
In order to directly establish whether the SNAP-induced delivery of AMPARs to the membrane surface is dependent on PICK1, we analyzed the effects of reducing the expression of the PICK1 protein. siRNAs directed against PICK1 were transfected into cultured neurons resulting in a 59.3 +/- 7.4% decrease of immunocytochemically detected dendritic PICK1 (Fig 5A). After 72 hours, neurons were treated with the NO donor SNAP followed by immunolabelling of surface GluR2-AMPARs. We found that reduction of basal PICK1 levels increased surface GluR2 levels (PICK11si/Ctrlsi 47 ± 10, n=4, p< 0.05; Fig. 5B, C right graph, but in left graph compare PICK1 si to Control). While SNAP induced an increase in surface AMPARs in neurons transfected with control siRNA, the SNAP-treated PICK1 siRNA transfected neurons showed no further changes in GluR2 at the membrane surface (Right Graph: SNAP/Ctrl 91.5± 30%; SNAP-PICK1si/PICK1si 20.6 ± 24.3%; n= 4; p< 0.01; Fig. 5B,C).
Figure 5. NO-mediated increase in surface AMPARs is dependent on dendritic PICK1 levels.
A, Images depict representative cells immunolabeled for PICK1 72 hours following transfection with control or PICK1 siRNA. Graph demonstrates the extent of reduction in dendritic PICK1 levels following siRNA treatments (n=4). B, Cells were transfected with either control siRNA or PICK1 siRNA for 72 hrs and subsequently treated with conditioned media ± SNAP (100 μM, 10 min). Images depict neurons immunolabeled for surface GluR2 AMPARs following treatment. C, Quantitation of A. Left graph shows the raw data, while the right graph depicts data normalized to appropriate control (n= 4; * p< 0.05, ** p< 0.01; Scale bar A: 2 μm). D, NMDA/low Mg2+ treatment induces an increase in NO production in hippocampal neurons. Graph depicts the quantitation of a colormetric assay showing elevated NO released from neurons treated with NMDA/low Mg2+. (n= 4; * p< 0.05)
NMDA/low Mg2+ elevates NO levels
Results presented thus far suggest that nitric oxide could mediate the unclustering of PICK1, similar to that induced by the strong activation of NMDARs (compare NMDA/low Mg2+ to SNAP-treated neurons). We therefore evaluated whether the generation of nitric oxide by activation of NMDARs is the mechanism by which NMDA causes PICK1 unclustering and surface AMPAR delivery. To determine whether NMDA/low Mg2+ treatment changes NO concentration in cultured hippocampal neurons we measured the conversion of nitrate to nitrite using a colormetric assay. We found that NMDA/low Mg2+ caused a drastic increase in the NO concentration from control levels (Ctrl 3.49 ± 1.66%, NMDA/low Mg2+ 11.28 ± 6.65%; n= 4, p< 0.05; Fig. 5D). These data indicate that higher NMDAR activation increases NO levels.
NMDA/low Mg2+-induced PICK1 unclustering and AMPARs insertion are mediated by NO.
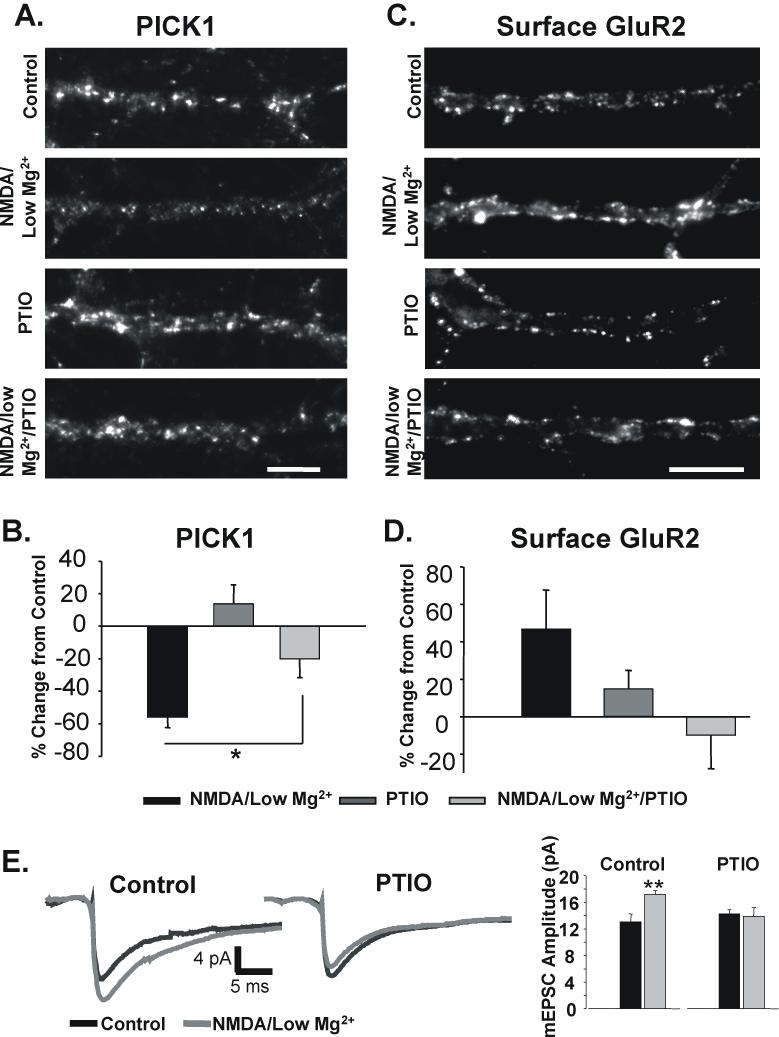
As NMDA/low Mg2+ treatment and NO seem to both uncluster PICK1, we wanted to determine if the NMDA/low Mg2+ effect is mediated by NO. We, therefore, performed a blocking experiment using a potent NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimi-dazoline-1-1-oxy-3-oxide (PTIO; 50 μM, 5min). While NMDA/low Mg2+ decreased PICK1 levels as before, it was unable to do so in the presence of PTIO (NMDA-low Mg2+/Ctrl -56 ± 6.4%; PTIO/Ctrl 14 ± 11%; NMDA-low Mg2+-PTIO/PTIO -20 ± 11.6%; n= 10; p< 0.05; Fig. 6A, B). Further experimentation revealed that PTIO similarly blocks the NMDA/low Mg2+-induced increase in surface AMPAR levels (NMDA/low Mg2+/Ctrl 46.8 ± 20.8%; PTIO/Ctrl 14.9 ± 9.8%; NMDA-low Mg2+-PTIO/PTIO -9.8 ± 17.8%; n= 5; Fig. 6C, D). We also investigated whether another stimulus known to induce NMDAR-dependent potentiation via AMPAR exocytosis in hippocampal cultures would similarly act to modulate PICK1 clustering (Lu et al. 2001). Treating cells with 200 μM glycine for 3 min resulted in a large decrease in the levels of clustered dendritic PICK1 which was also blocked by PTIO (Glycine -46.7 +/- 7.6% change in PICK1 labeling from control, n= 8; Glycine/ PTIO 0.3 +/- 14.9 % change from control, n=4, Supplementary Fig1).
Figure 6. NO production mediates the NMDAR-induced decrease in dendritic PICK1 and increase in surface AMPAR expression.
A, NO signaling was blocked with the NO scavenger PTIO in control and NMDA/low Mg2+-treated neurons prior to immunocytochemical analysis of PICK1 levels (50 μM; 5 min). Fluorescent images show dendrites immunostained for PICK1. B, Quantitation of experiments in A reveal that PTIO blocks the NMDAR-induced decrease in dendritic PICK1 clustering (n= 10; * p< 0.05). C, Examples of dendrites from neurons labeled for surface GluR2-AMPARs in control and NMDA/low Mg2+ cells treated with and without PTIO. D, Quantitation of experiments as in C (n= 5, Scale bar = B: 5 μm; D: 3 μm) E, The amplitude of mEPSCs was measured in untreated neurons (left traces, black) and in neurons 15 minutes after a 1 minute application of NMDA/low Mg2+ (left traces, grey). Experiments were repeated in neurons also treated with PTIO (right traces). Recordings of control and agonist-treated neurons were made on the same day from the same batch of neurons. Graph depicts average amplitude of mEPSCs recorded under the four treatment conditions (n=5 slips, 12 cells, ** p< 0.01)
To test whether these observed NO-dependent changes in surface AMPARs functionally impact synaptic responsiveness in hippocampal neurons, we examined the effects of NMDA/low Mg2 treatment on miniature excitatory synaptic currents (mEPSCs). The amplitudes of mEPSCs was significantly increased in neurons treated with NMDA/low Mg2 (Control 13.1+/- 1.2 pA, NMDA/low Mg2+ 17.2 +/- 0.63 pA, n= 5 slips, 12 cells, p< 0.01, Fig 6E). The increase was not observed in neurons pretreated with PTIO (PTIO alone 14.28+/- 0.63 pA, NMDA/low Mg2+ / PTIO 13.9 +/- 1.3 pA, n= 5, 12 cells, n.s., Fig 6E). Together, these data demonstrate that the NMDAR-dependent unclustering of PICK1 and the consequent AMPAR trafficking and synaptic potentiation are mediated by the generation of nitric oxide.
Discussion
This study shows that NO can effect the plasma membrane expression of a population of receptors regulated by PICK1. Our data further elucidates a mechanism underlying the surface delivery of AMPARs following NMDAR activation. NMDAR stimulation enhances nitric oxide levels in hippocampal neurons and induces an increase in the S-nitrosylation of NSF. Consistent with work by Huang et al. (Huang et al. 2005), this modification of NSF is accompanied by an increase in the binding of NSF to GluR2. We now find that these events are accompanied by an unclustering of PICK1, presumably from endosomes in neuronal dendrites (Sossa et al. 2006). A link between NO generation and the effects on PICK1 were confirmed by NO donors, which alone induce the unclustering of dendritic PICK1 and the surface delivery of AMPARs. As PICK1 is also known to be involved in AMPAR endocytosis, it is possible that the increase in surface AMPARs with PICK1 unclustering reflects not increased delivery, but rather a decrease in the constitutive endocytosis of AMPARs. This would lead to an accumulation of cycling AMPARs at the membrane surface. However, we find that NO-induced unclustering of PICK1 does not reduce the rate of constitutive endocytosis for GluR2 AMPARs, suggesting that the changes in PICK1 are influencing the overall delivery of surface AMPARs (Supplementary Figure 2).
The apparent surface delivery of AMPARs by NO donors is dependent on the expression of PICK1 confirming the link between nitric oxide and PICK1 in the redistribution of AMPARs to the membrane. Finally, the NMDAR-dependent unclustering of PICK1 and the insertion of AMPARs are blocked by scavengers of NO. Taken together, we conclude that the stimulation of NMDARs triggers NSF-dependent unclustering of dendritic PICK1, the dissociation of PICK1 from GluR2, and finally the movement of these receptors to the membrane surface.
We find that the interaction of NSF and SNAP is critically involved in NMDAR-induced PICK1 unclustering. The binding of SNAP to NSF increases the ATPase activity of NSF, which is known to be essential for the unbinding of PICK1 from GluR2 (Hanley et al. 2002). Disruption of the association between NSF and SNAP with an interfering peptide significantly inhibited the NMDA/low Mg2+-induced redistribution of PICK1 suggesting the importance of this interaction. However, the incomplete blockade we observe indicates that the basal ATPase activity of NSF that is independent of SNAP is likely to be sufficient to mediate some PICK1 unclustering.
In the central nervous system, NO is synthesized by the nitric oxide synthase (NOS), a calcium/calmodulin-dependent enzyme. NOS complexes with NMDARs via an interaction with PSD-95 (Christopherson et al. 1999), which likely facilitates calcium influx-mediated NO synthesis (Bredt et al. 1989). We have previously shown that NMDA/low Mg2+ treatment allows for a high level calcium entry, and that the unclustering of PICK1 is dependent on intracellular calcium concentrations. If this calcium is acting directly through the activation of NOS, inhibition of calmodulin activity would be expected to block NMDAR-mediated PICK1 unclustering. However, we found that calmodulin inhibitors themselves affect the basal clustering of PICK1 in dendrites (data not shown). This suggests that calmodulin-dependent effectors may influence PICK1 clustering at multiple levels, although we can not confirm its role in activating NOS in our experiments.
The role of NO in NMDAR-dependent long-term plasticity in the CA1-region of the hippocampus remains in dispute. At one time evidence suggested that long-term plasticity at CA1 hippocampal neurons was expressed via the retrograde action of postsynaptically generated nitric oxide (NO) on presynaptic terminals (O’Dell et al. 1991; Schuman et al. 1991), where it increases cGMP levels and influences vesicular release (Stevens 1988). Additional evidence suggests that NO can act presynaptically to drive synaptic depression (Gage et al. 1997). However, subsequent debate challenged whether NO is actually involved in the long-term potentiation (LTP) of synaptic responses as it was found that LTP was obtainable in the absence of NOS (O’Dell et al. 1994). Another study furthered the controversy by showing that LTP is blocked in independently created NOS mutant mice (Kantor et al. 1996). While the role for NO in potentiation remains unresolved, intriguing recent evidence shows a role for postsynaptic NO following chemical LTP, where S-nitrosylation of NSF increases AMPAR trafficking to the membrane surface (Huang et al. 2005). This together with our findings suggests that NO may contribute to the potentiation of synaptic responses.
Our results lie in apparent conflict with studies suggesting no role for NO in LTP. This conflict may be explained by the existence of multiple mechanisms contributing to synaptic potentiation. Standard models of NMDAR-LTP suggest that insertion of GluR1-containing receptors, which do not bind NSF, mediate LTP in a CaMKII-dependent manner (Hayashi et al. 2000; Passafaro et al. 2001; Plant et al. 2006). These receptors are then thought to be replaced by cycling GluR2/3 AMPARs (Plant et al. 2006). Our data showing a preferential increase in surface GluR2 over GluR1 receptors could reflect the later effects of this process of receptor exchange. Alternatively, as classically defined NMDAR-LTP likely occurs independently of NO-mediated effects, the potentiation described here may reflect a different form of plasticity. The release of NO by NMDAR activation may mediate the delivery of previously internalized GluR2/3-containing (GluR1-lacking) AMPARs held intracellularly by PICK1; a process more consistent with a model of dedepression. As NMDAR-dependent LTP and dedepression can be elicited by the same stimuli, the preexisting state of synapses (Montgomery et al. 2004) may determine whether or not synaptic potentiation elicited is NO-dependent.
Here, we provide evidence that NO contributes to changes in synaptic strength, but also offer an explanation as to why previous attempts to link NO to LTP have often failed. Our results describe a model by which cycling AMPARs, held in intracellular pools, are rapidly delivered to the membrane surface by a NO-mediated activation of NSF. This work is consistent with PICK1 suppressing the availability of select AMPARs for surface expression, disrupted only when S-nitrosylated NSF chaperones receptors towards the plasma membrane. This PICK1-regulated process may likely underlie different forms of potentiation.
Supplementary Material
Supplementary Figure 1
Activation of synaptic NMDARs with glycine induces unclustering of dendritic PICK1 dependent on NO production
A, NO signaling was blocked with the NO scavenger PTIO in control and glycine-treated neurons prior to immunocytochemical analysis of PICK1 levels. (Glycine: 200 ¼M for 3 min). Fluorescent images show dendrites immunostained for PICK1. B, Quantitation of experiments in A reveal that PTIO blocks the co-agonist induced decrease in dendritic PICK1 clustering.
Supplementary Figure 2
NO does not reduce the basal rate of GluR2 endocytosis
A, Live hippocampal neurons were labeled with an antibody recognizing an extracellular epitope on the GluR2 subunit. Upon wash off of the antibody cells were returned to 37°C for 0, 15 or 30 minutes. Cells were fixed and processed to fluorescently immunolabel only receptors remaining the membrane surface. Images depict labeling of GluR2 AMPARs remaining on the surface of control and SNAP treated neurons (SNAP added 20 minutes before and throughout timecourse). B, The extent of constitutive receptor endocytosis was plotted for control and SNAP-treated neurons as a ratio of surface labeling at 15 and 30 minutes normalized to that at 0 minutes. The release on NO did not reduce the basal rate of GluR2 endocytosis.
Acknowledgements
This work was supported by NIH/NINDS NS 049661 and the Whitehall Foundation. KGS was supported by NIH Training Grant 5T32NS007439.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Braithwaite SP, Meyer G, Henley JM. Interactions between AMPA receptors and intracellular proteins. Neuropharmacology. 2000;39(6):919–30. doi: 10.1016/s0028-3908(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989;86(22):9030–3. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274(39):27467–73. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51(3):339–49. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Khatri L, Hanson PI, Ziff EB. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron. 2002;34(1):53–67. doi: 10.1016/s0896-6273(02)00638-4. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Huang Y, Man H, Sekine-Aizawa Y, Juluri HY,K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-Nitrosylation of N-Ethylmaleimide Sensitive Factor Mediates Surface Expression of AMPA Receptors. Neuron. 2005;46(4):533–40. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001(86):PL1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Lanzrein M, Stary SJ, Sandoval GM, Smith WB, Sullivan BM, Davidson N, Schuman EM. A role for endothelial NO synthase in LTP revealed by adenovirus-mediated inhibition and rescue. Science. 1996;274(5293):1744–8. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5(10):771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279(5349):399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29(1):243–54. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. Discrete synaptic states define a major mechanism of synapse plasticity. Trends Neurosci. 2004;27(12):744–50. doi: 10.1016/j.tins.2004.10.006. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991;88(24):11285–9. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994;265(5171):542–6. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4(9):917–26. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9(5):602–4. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254(5037):1503–6. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–6. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Sossa KG, Court BL, Carroll RC. NMDA receptors mediate calcium-dependent, bidirectional changes in dendritic PICK1 clustering. Mol Cell Neurosci. 2006;31(3):574–85. doi: 10.1016/j.mcn.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Stevens CF. Neurotransmission. A relaxing factor in the brain. Nature. 1988;336(6197):308–9. doi: 10.1038/336308a0. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25(32):7342–51. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Activation of synaptic NMDARs with glycine induces unclustering of dendritic PICK1 dependent on NO production
A, NO signaling was blocked with the NO scavenger PTIO in control and glycine-treated neurons prior to immunocytochemical analysis of PICK1 levels. (Glycine: 200 ¼M for 3 min). Fluorescent images show dendrites immunostained for PICK1. B, Quantitation of experiments in A reveal that PTIO blocks the co-agonist induced decrease in dendritic PICK1 clustering.
Supplementary Figure 2
NO does not reduce the basal rate of GluR2 endocytosis
A, Live hippocampal neurons were labeled with an antibody recognizing an extracellular epitope on the GluR2 subunit. Upon wash off of the antibody cells were returned to 37°C for 0, 15 or 30 minutes. Cells were fixed and processed to fluorescently immunolabel only receptors remaining the membrane surface. Images depict labeling of GluR2 AMPARs remaining on the surface of control and SNAP treated neurons (SNAP added 20 minutes before and throughout timecourse). B, The extent of constitutive receptor endocytosis was plotted for control and SNAP-treated neurons as a ratio of surface labeling at 15 and 30 minutes normalized to that at 0 minutes. The release on NO did not reduce the basal rate of GluR2 endocytosis.