Abstract
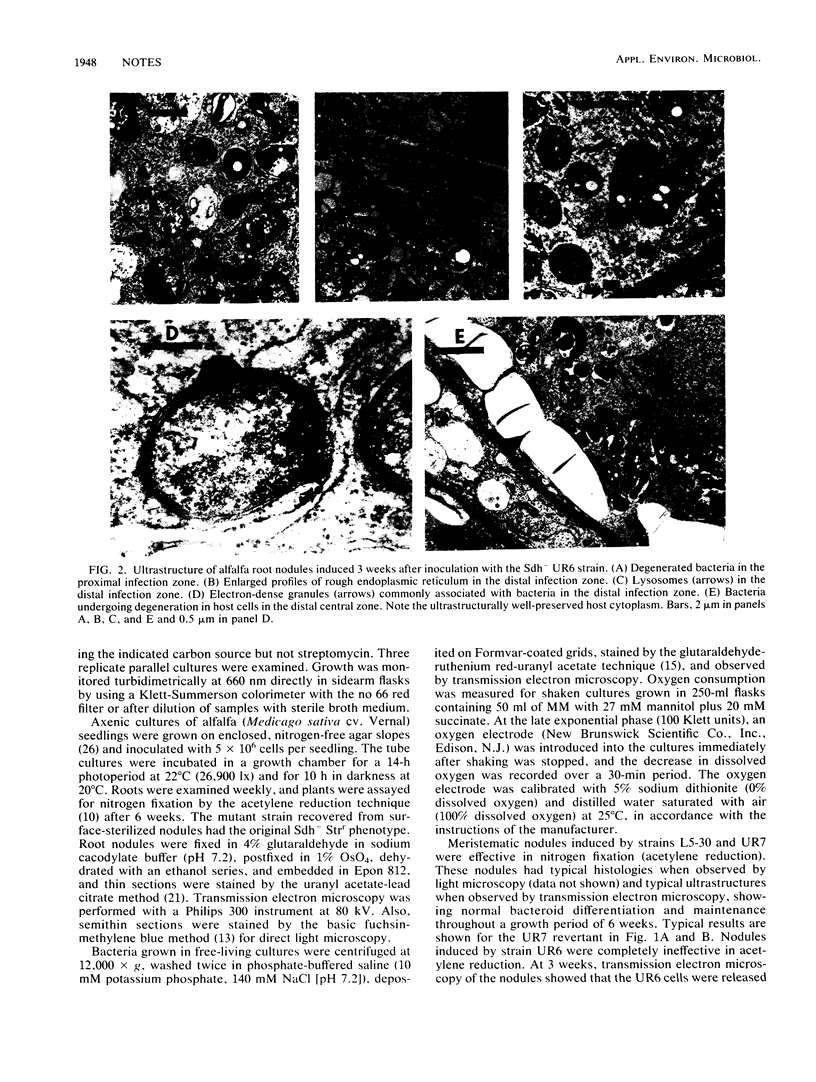
Transmission electron microscopy was used to study the cellular morphologies of a wild-type Rhizobium meliloti strain (L5-30), a nitrogen fixation-ineffective (Fix-) succinate dehydrogenase mutant (Sdh-) strain, and a Fix+ Sdh+ revertant strain within alfalfa nodules and after free-living growth in a minimal medium containing 27 mM mannitol plus 20 mM succinate. The results showed a requirement of succinate dehydrogenase activity for symbiotic differentiation and maintenance of R. meliloti bacteroids within alfalfa nodules and for succinate-induced cellular pleomorphism in free-living cultures. Also, the Sdh- strain had a 3.5-fold lower rate of oxygen consumption in the defined medium than did the wild type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Gibson A. H., Dudman W. F. Nitrogenase activity and respiration of cultures of Rhizobium spp. with special reference to concentrations of dissolved oxygen. Biochim Biophys Acta. 1976 Aug 24;444(1):164–174. doi: 10.1016/0304-4165(76)90233-6. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Succinate transport in Rhizobium leguminosarum. J Bacteriol. 1981 Oct;148(1):193–202. doi: 10.1128/jb.148.1.193-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J Bacteriol. 1983 Jun;154(3):1403–1413. doi: 10.1128/jb.154.3.1403-1413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol A., Arias A., Cerveñansky C., Martínez-Drets G. Succinate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1982 Sep;151(3):1621–1623. doi: 10.1128/jb.151.3.1621-1623.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Succinate uptake and related proton movements in Escherichia coli K12. Biochem J. 1975 Dec;152(3):647–654. doi: 10.1042/bj1520647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- McAllister C. F., Lepo J. E. Succinate transport by free-living forms of Rhizobium japonicum. J Bacteriol. 1983 Mar;153(3):1155–1162. doi: 10.1128/jb.153.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Lyttleton P., Robertson J. G. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4284–4288. doi: 10.1073/pnas.78.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall I., Cole M. Organic Acid Metabolism by Isolated Rhizobium japonicum Bacteroids. Plant Physiol. 1978 May;61(5):787–790. doi: 10.1104/pp.61.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. A micromethod for the purification and quantification of organic acids of the tricarboxylic acid cycle in plant tissues. Anal Biochem. 1979 May;95(1):311–315. doi: 10.1016/0003-2697(79)90221-5. [DOI] [PubMed] [Google Scholar]
- Urban J. E., Davis L. C., Brown S. J. Rhizobium trifolii 0403 Is Capable of Growth in the Absence of Combined Nitrogen. Appl Environ Microbiol. 1986 Nov;52(5):1060–1067. doi: 10.1128/aem.52.5.1060-1067.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. E., Dazzo F. B. Succinate-Induced Morphology of Rhizobium trifolii 0403 Resembles That of Bacteroids in Clover Nodules. Appl Environ Microbiol. 1982 Jul;44(1):219–226. doi: 10.1128/aem.44.1.219-226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. E. Nondividing, Bacteroid-Like Rhizobium trifolii: In Vitro Induction Via Nutrient Enrichment. Appl Environ Microbiol. 1979 Dec;38(6):1173–1178. doi: 10.1128/aem.38.6.1173-1178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]






