Abstract
DNA immunization can result in the induction of Ag-specific cellular and humoral immune responses and in protective immunity in several Ag systems. To evaluate the utility of DNA-based immunization as a potential cancer treatment strategy, we employed an experimental murine tumor, CT26, expressing the model tumor-associated Ag, β-galactosidase (β-gal), designated CT26.CL25. A plasmid expressing β-gal (pCMV/β-gal) administered by particle-mediated gene delivery to the epidermis using a hand-held, helium-driven “gene gun” induced β-gal-specific Ab and lytic responses. Immunization with this construct prevented the growth of pulmonary metastatic tumor, and the adoptive transfer of splenocytes generated by pCMV/β-gal in vivo immunization and cultured in vitro with the β-gal876–884 immunodominant peptide reduced the number of established pulmonary nodules. DNA immunization alone had little or no impact on the growth of established lung metastases. To enhance the function of DNA immunization for active immunotherapy, a panel of cytokines was added as adjuvants following DNA administration. Significant reduction in the number of established metastases was observed when human rIL-2, mouse rIL-6, human rIL-7, or mouse rIL-12 were given after DNA inoculation; mouse rIL-12 as an adjuvant had the most profound effect. These findings suggest that the cytokines involved in the activation and expansion of lymphocyte populations may improve the therapeutic effects of DNA immunization. Given the ease with which plasmid DNA can be prepared to high purity for safe use in humans with infectious diseases and cancers, DNA immunization administered together with cytokine adjuvant may be an attractive alternative to recombinant viral vaccines.
The induction of antitumor immunity, in part, involves CTL responses (1-3). The recent cloning of genes encoding tumor-associated Ag (TAA)3 recognized by CTL makes it possible to design Ag-specific recombinant viral and nonviral vectors that allow for control over parameters such as the quantity and kinetics of expression, the intracellular compartment into which the TAA are expressed, and the tissues or cell types that are used to express TAA in vivo (4-9). For example, several viruses including recombinant vaccinia virus, fowlpox virus, and adenovirus encoding model TAAs have been shown to express Ags within the cytoplasm of infected cells, resulting in the induction of tumor immunity (10-12, 12a). However, immunization with live attenuated or recombinant viruses, although potent, may also pose safety problems due to their infectious nature (13). In addition, immune responses against heterologous protein may be reduced when the host has had previous exposure to the virus such as has been observed with vaccinia virus (14-16).
DNA-based immunization is an attractive nonviral alternative for cancer immunotherapy. DNA vaccination is accomplished by the expression of inoculated bacterial plasmid DNA encoding the foreign gene of interest accompanied by a mammalian promoter/enhancer, and other sequences such as Kozak’s consensus sequence and leader sequences that enable the gene to be expressed within mammalian cells utilizing host machinery (17-19). In this report, a hand-held helium-powered device was used to achieve the direct intracellular delivery of DNA-coated gold particles to the epidermis. Following delivery, the DNA redissolves in the aqueous environment of the cytoplasm or nucleus and is then available for expression (20-25). Alternatively, skeletal muscle cells have demonstrated the ability to take up and express DNA for approximately the lifetime of the mouse without any specific delivery system (17, 18, 26-28). However, epidermal gene gun immunization of DNA may be more efficient than intramuscular immunization at eliciting similar immune responses (22, 25).
Both DNA-based approaches have been shown to successfully induce both humoral and cellular immunity in many Ag systems (22-25, 29-34). Gene gun delivery of DNA encoding human growth hormone (hGH) resulted in specific Ab responses that correlated with particle delivery to the mouse epidermis (23, 29). Intramuscular inoculation of plasmid DNA encoding the influenza A viral nucleoprotein could induce protective humoral and cellular immunity (30). Protective immunity after DNA-based immunization has also been observed using gp160 and rev proteins from HIV I, and H1 Ag from influenza, circumsporozoite protein from malaria, and nucleoprotein from lymphocytic choriomeningitis virus (22, 31-34). In cancer immunotherapy, plasmid constructs encoding either the full length cDNA for carcinoembryonic Ag (CEA) or HIV-1 envelope protein, gp160, have been shown to protect mice from subsequent challenge with syngeneic tumors expressing these model Ags (35-37).
To the best of our knowledge, no group has shown therapeutic activity against established tumors using either “naked” DNA injection or gene gun immunization. In this study, we used the murine colon adenocarcinoma (CT26.WT) transfected with the gene for the model TAA, β-gal (CT26.CL25) (12), to test the antitumor potential of a plasmid DNA-based vaccine using a gene gun delivery system. Here, we show that DNA-based vaccines enhanced with the systemic administration of rhIL-2, rmIL-6, rhIL-7, or rmIL-12 may have utility in the active immunotherapy of cancer.
Materials and Methods
Tumor cell lines and animals
CT26.WT is a clone of the N-nitroso-N-methylurethan-induced BALB/c (H-2d) undifferentiated colon carcinoma (38). Following transduction with a retrovirus encoding the LacZ gene, CT26.WT was subcloned to generate the β-gal-expressing cell line CT26.CL25 (12). All tumor cell lines were grown and maintained in complete medium (CM) containing RPMI 1640, 10% heat-inactivated FCS (both from Biofluids, Rockville, MD), 0.03% fresh L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin (all from National Institutes of Health, Media Unit, Bethesda, MD), and 50 μg/ml gentamicin sulfate. CT26.CL25 was grown in the presence of 400 μg/ml G418 (Life Technologies, Inc., Grand Island, NY). Female BALB/c mice, 6 to 10 wk old, were obtained from the Animal Production Colonies, Frederick Cancer Research Facility. National Institutes of Health (Frederick, MD).
Plasmid preparations
A plasmid encoding the Escherichia coli Lac2 gene under the control of the human CMV intermediate-early promoter, designated pCMV/β-gal, was kindly provided by J. Haynes (Agracetus, Middleton, WI). Plasmids expressing either human growth hormone (pCMV/hGH), provided by J. Haynes, or the nucleoprotein from influenza A (A/pR/8/34) (pCMV/NP) under the control of the CMV intermediate-early promoter were used as control vectors in this study. Constructs were transformed into E. coli DH5 α-competent cells (Life Technologies, Inc.) and grown in L-broth with 100 μg/ml ampicillin as described (39). Closed circular plasmid DNA was isolated using Wizard Maxipreps DNA purification kits (Promega Corp., Madison, WI). The 260/280 ratios ranged from 1.8 to 2.0.
Peptides
The synthetic peptide TPHPARIGL, representing the naturally processed H-2 Ld-restricted epitope spanning 876–884 of β-gal (40) and the influenza NP147–155 peptide, TYQRTRALV, presented by H-2Kd, were synthesized by Peptide Technologies (Washington, DC) to a purity of greater than 99% as assessed by HPLC and amino acid analysis.
Gene gun delivery of DNA
Plasmid DNA was affixed to gold particles by adding 10 to 50 mg of 0.95-μm-diameter gold powder (kindly provided by Agracetus, Middleton, WI) to a 1.5-ml centrifuge tube containing 100 μl of 0.1 M spermidine (Sigma Chemical Co., St. Louis, MO). Plasmid DNA and gold were coprecipitated by the addition of 200 μl of 2.5 M CaCl2, during vortex mixing as previously described (24). After settling for 10 min, the precipitate was washed with absolute ethanol to remove H2O and resuspended at either 3.5 mg gold/ml or 7.0 mg/ml of ethanol, which would result in 0.25 mg or 0.5 mg of gold particles per shot, respectively. While the amount of gold per shot remained constant, the total amount of DNA per shot ranged from 0.001 to 1.0 μg per shot. Animals were anesthetized with 200 μl of a 9% solution of sodium pentobarbital while abdominal areas were shaved. DNA-coated gold particles were delivered into abdominal epidermis using the hand-held, helium-driven Accell gene delivery system (kindly provided by Agracetus). Each animal received 2 to 10 nonoverlapping deliveries per immunization (as designated below), at a pressure of 400 psi of helium.
Enzyme-Linked lmmunosorbent Assay
BALB/c mice were immunized two times at 2-wk intervals with 0.001 to 1.0 μg of either pCMVIP/β-gal or pCMV/NP using the gene gun. Serum samples were collected 2 wk following the second immunization and analyzed for the presence of anti-β-gal Abs by ELISA. Specifically, microtiter plates were dried overnight at 37°C in a nonhumidified incubator with 200 ng/well/50 μl of either purified β-gal or control Ag, ovalbumin (both obtained from Sigma Chemical Co., St. Louis, MO). Incubation of 100 μl of 5% BSA in PBS on each well for 1 h to prevent nonspecific Ab binding was followed by a second 1-h incubation with 50 μl of fivefold dilutions (starting at 1:100) of test sera or control anti-β-gal murine mAb (starting at 100 ng/50 μl). After washing with 1% BSA in PBS, horseradish peroxidase-conjugated sheep anti-mouse IgG F(ab′)2, fragments (1:3000) (Amersham International, Amersham, UK) were added for 1 h at 37°C to detect Abs immobilized on the wells. The resulting complex was detected by the chromogen, o-phenylenediazamine (Sigma Chemical Co.). Absorbance was read on a Titertek Multiskan Plus reader (Flow Laboratories, McLean, VA) using a 490-nm pore diameter filter. Concentrations of β-gal-specific Ab in serum samples were estimated from the mAb standard curve and expressed as μg/ml.
Effector cells
Primary lymphocyte populations were generated by immunization with different amounts of purified pCMV/β-gal or pCMV/NP. Secondary in vitro effector populations were generated by harvesting spleens of mice 14 days after immunization and culturing single cell suspensions of splenocytes in T-75 flasks (Nunc, Roskilde, Denmark) at a density of 3.0 × 106 cells/ml with 1 μg/ml antigenic peptide at a total volume of 30 ml of CM containing 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate (both from Biofluids), and 5 × 10−5 M 2-ME (Life Technologies, Inc., Rockville, MD) in the absence of IL-2. Six days later splenocytes were harvested and washed in CM before testing in a 51Cr release assay or for transfer to tumor-bearing animals.
51Cr release assay
Six-hour 51Cr release assays were performed as previously described (41). Briefly, 2 × 106 target cells were incubated in 0.2 ml of CM labeled with 200 μCi of Na51 CrO4 for 90 min. Peptide-pulsed CT26.WT were incubated with 1 μg/ml (approximately 1 μM) antigenic peptide during labeling as previously described (42). Target cells were then mixed with effector cells for 6 h at 37°C at the effector to target ratios indicated. The amount of 51Cr released was determined by gamma counting and the percentage of specificlysis was calculated as follows.
In vivo studies
For in vivo prevention studies, mice were immunized with different amounts of pCMV/β-gal or either control pCMV/hGH or pCMV/NP. Fourteen days later, mice were challenged i.v. with 2 × 105 tumor cells (43). Mice were killed on day 17 and randomized before counting lung metastases in a blind fashion as previously described (43).
Adoptive immunotherapy experiments
BALB/c mice were injected i.v. with 2 × 105 CT26.WT (β-gal−) or CT26.CL25 (β-gal+) cultured tumor cells in 0.5 ml of HBSS to induce pulmonary metastases. On day 3, tumor-bearing mice were treated with an i.v. injection of various effect or cells at 5 × 106 cells/dose. Specifically, for the generation of effector cells, mice were immunized with 1 μg of pCMV/β-gal or pCMV/NP. Each mouse received two 0.25-mg shots of gold loaded with 0.5 μg of DNA. Two weeks later, splenocytes were harvested and cultured for 6 days in CM plus 5 × 10−5 M 2-ME, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate with 1 μg/ml of either β-ga1876–884 or NP147–155 synthetic peptide. On day 17 after tumor injection, mice were killed and pulmonary metastases were enumerated in a coded, blind fashion. When metastases exceeded 250, they were deemed too numerous to count.
In vivo treatment studies
BALB/c mice were challenged with 5 × 105 CT26.WT or CT26.CL25 tumor cells i.v. to establish pulmonary metastases. On day 2, mice were immunized with 10 μg of pCMV/β-gal, pCMV/NP DNA, or no DNA. Each mouse received 10 nonoverlapping shots of gold (0.5 mg each) delivering l μg of DNA. On day 12, the mice were killed and metastatic lung nodules were enumerated in a randomized, blind manner. As a positive control, a group of mice was included that received a recombinant vaccinia virus encoding the β-gal (VJS6), plus the exogenous administration of the cytokine, IL-2 (15,000 U, twice daily (BID) for 5 days) as previously reported (44).
Intraperitoneal treatments of various cytokines began 18 to 24 h following DNA administration and continued daily for 3 to 7 days depending on the cytokine. Specifically, one group of mice received 15,000 Cetus U of rhIL-2, BID, for 5 days (Chiron Corp., Emeryville, CA) (44). A second group of mice received treatments of 0.5 μg of rmIL-6, BID, for 3 days (Peprotech, Inc., Rocky Hill, NJ). A third group received 5 μg of rhIL-7, BID, for 7days (Peprotech). A fourth received 1.0 μg of rmIL-12 once daily (QD) for 5 days (Genetics Institute, Boston, MA). In a screening experiment of many cytokines (data not shown), rmIL-4 (5 μg, BID, 7 days), rmIL-10 (1.0 μg, QD, 7 days), and GM-CSF (1.0 μg, QD, 5 days) were also assayed for adjuvant activity (Peprotech). These parameters were determined from previous reports demonstrating antitumor immune responses from their exogenous administration (45-50). These doses of cytokine alone were shown to have little or no effects on the growth of CT26.WT of CT26.CL25.4
Statistical analysis
Because lungs that contained >250 pulmonary metastases were deemed too numerous to count, the data do not follow a normal distribution. Thus, statistical evaluation of the data was performed using the nonparametric two-tailed Kruskal-Wallis test.
Results
DNA-based vaccine elicits Ag-specific humoral and cellular immunity
To study the induction of Ag-specific humoral immunity using a DNA-based vaccine, mice were immunized with a plasmid cDNA encoding the model TAA β-gal (pCMV/β-gal). Mice immunized and boosted with gold particles coated with as little as 0.01 μg of pCMV/β-gal developed β-gal-specific Ab responses (Fig. 1). In contrast, gold particles coated with 1.0 μg of the control plasmid pCMV/NP failed to elicit β-gal-specific Ab. No reactivity was observed against a control Ag, ovalbumin, confirming the specificity of the humoral immune response (data not shown).
FIGURE 1.
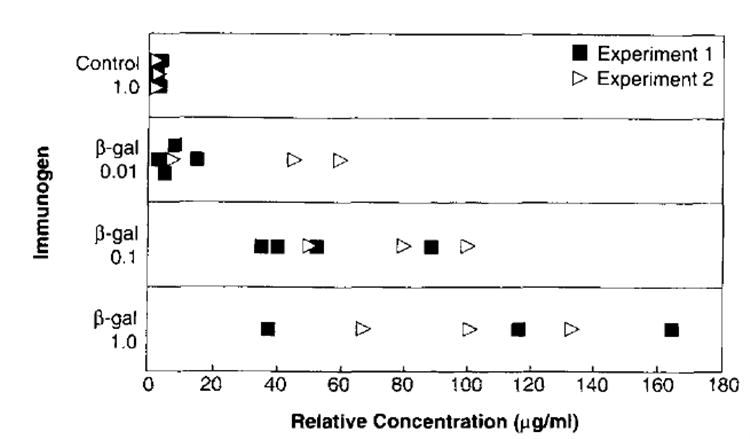
Induction of humoral immunity elicited with gene gun vaccination of pCMV/β-gal. BALB/c mice (three per group) were immunized two times at 2-wk intervals into the epidermis; each immunization consisted of four shots of 0.25 mg of gold delivering a total of either 1.0 μg of control pCMV/NP, or 0.01 μg, 0.1 μg, or 1.0 μg of pCMV/β-gal. Fourteen days following the boost, sera were tested by ELISA for the presence of Abs against recombinant β-gal protein. The relative concentration of Abs reactive against β-gal were calculated from a standard curve of an anti-β-gal mAb and expressed as μg/ml .
To test whether a CTL response could be elicited against β-gal expressed by DNA immunization, BALB/c mice were inoculated once with varying quantities of pCMV β-gal or control vector, pCMV/NP. Splenocytes from mice that were injected with as little as 0.1 μg of pCMV/β-gal and then restimulated in vitro with the β-gal876–884 peptide lysed the β-gal-transfected tumor, CT26.CL25 (Fig. 2). CT26.WT pulsed with the immunodominant peptide β-gal876–884 was also recognized by the pCMV/β-gal-immune splenocytes generated with as little as 0.01 μg of DNA. Pulsed cells in this case appeared to be more sensitive to the lytic cells than the transfected cells, CT26.CL25. Unpulsed CT26.WT was not significantly lysed, demonstrating the specificity of the lytic response. Splenocytes derived from mice immunized with pCMV/NP and cultured in vitro in an identical manner as described above failed to lyse CT26.WT. CT26.WT pulsed with the β-gal876–884 or CT26.CL25. Together, these data indicated that DNA administered with the gene gun was able to elicit specific humoral and cellular immunity against the model tumor Ag, β-gal.
FIGURE 2.
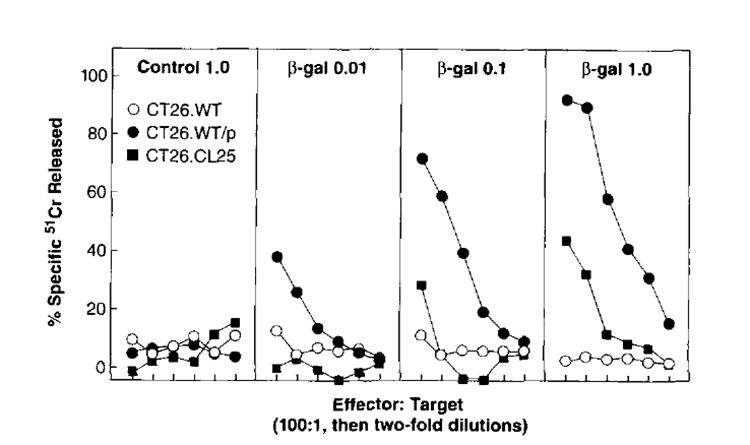
Secondary in vitro TCD8+ induced by immunization with gene gun vaccination of pCMV/β-gal. BALB/c mice were immunized one time in the epidermis. Each immunization consisted of four shots of 0.25 mg of gold delivering a total of 1.0 μg pCMV/NP control DNA, 0.01 μg of pCMV/β-gal, 0.1 μg of pCMV/β-gal, or 1.0 μg of pCMV/β-gal. Fourteen days later pooled splenocytes (two mice per group) were restimulated in vitro with 1 μg of β-gal876–884 peptide for 6 days and then assayed for specific lytic activity in a 51Cr release assay against CT26.WT(β-gal−), CT26.CL25 (β-gal+), or CT26.WT pulsed with β-gal876–884 peptide target cells. These experiments have been repeated two times with similar results.
Prophylactic DNA vaccine protects mice from intravenous tumor challenge
To determine whether humoral and cellular responses observed in vitro correlated with in vivo antitumor activity, mice were immunized with the DNA vaccine and assayed for the growth of a subsequent i.v. tumor challenge. Only the mice that received pCMV/β-gal showed significant responses compared with the mice that were inoculated with control DNA (Fig. 3). In pooled results from three experiments, almost complete protection was observed with 1.0 μg of pCMV/β-gal; 19 of 20 pairs of lungs from immunized mice were devoid of any detectable tumor. At a 10-fold lower dose, 16 of 20 pairs of lungs were completely free of disease. With a dose of 0.01 μg of DNA immunized, the protective effects began to wane, with only 6 of 20 mice disease free. This correlated with decreasing amounts of Ab and CTL activity observed in the mice that received 0.01 μg of DNA (Figs. 1 and 2). In addition, mice immunized with pCMV/β-gal and then challenged with β-gal− CT26.WT were not protected from tumor growth (Fig. 3). Therefore, these data suggest that the protection from tumor following gene gun inoculation of DNA encoding a TAA is Ag specific both at the level of the immunizing plasmid and at the level of Ag expression by tumor.
FIGURE 3.
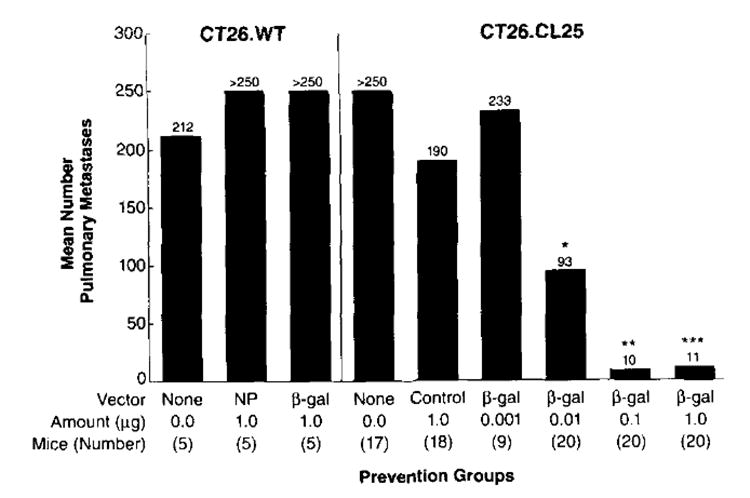
Immunization with DNA vaccine prevents the growth of i.v. tumors. On day 0, BALB/c mice were immunized one time in the epidermis. Each immunization consisted of five shots of 0.25 mg of gold delivering 1.0, 0.10, or 0 .01 μg of pCMV/β-gal or 1.0 μg of control DNA alone. Fourteen days later, mice (5 to 10 per group) were challenged, i.v., with 2.0 × 105 CT26.CL25 (β-gal+) or CT26.WT (β-gal−) tumor cells. On day 17, lungs were harvested and tumor nodules were enumerated in a blind fashion. Statistical analysis was carried out using the nonparametric Kruskal-Wallis test; therefore error bars are not shown. This graph represents a summary of all the data from three separate experiments. In the first experiment, the control DNA utilized was pCMV/hGH, while in the remaining experiments, pCMV/NP was used.
Adoptive transfer of splenocytes generated using DNA vaccine mediate reduction of established pulmonary metastases
To ascertain whether the effector cells elicited by gene gun immunization were active in in vivo adoptive immunotherapy, we used a 3-day lung metastasis model (43). In these experiments, BALB/c mice were given i.v. injections with either CT26.CL25 (β-gal+) or CT26.WT (β-gal−) tumor cells. On day 3, tumor-bearing mice were treated with splenocytes from donor mice that had received gene gun immunization with either pCMV/β-gal or pCMV/NP, and then subsequent ex vivo incubation with either β-gal876–884 or control NP147–155 peptide.
Effector cells generated from mice immunized with pCMV/β-gal and cultured with β-gal876–884 peptide completely cleared the lungs of mice bearing 3-day-old pulmonary metastases (Fig. 4). By contrast, lungs from CT26.CL25-bearing mice that received either no splenocytes, splenocytes from pCMV/β-gal-immunized mice stimulated with the irrelevant synthetic peptide (NP147–155), or splenocytes from pCMV/NP-immunized mice stimulated with the β-gal876–884 peptide, contained >250 metastases. In addition, the splenocytes induced by pCMV/β-gal and stimulated with β-gal876–884 peptide were not effective at eliminating metastases in mice bearing the Ag-negative tumor, CT26.WT (Fig. 4), demonstrating the in vivo specificity for the β-gal Ag. Similar results were observed in a repeat experiment. Thus, the induction of immune splenocytes for adoptive transfer was Ag specific, and the therapeutic activity of these cells was specific for Ag expression by the tumor. The potential therapeutic implications of this approach are clear, since this strategy involved not just the prevention of disease, but the treatment of established metastases.
FIGURE 4.
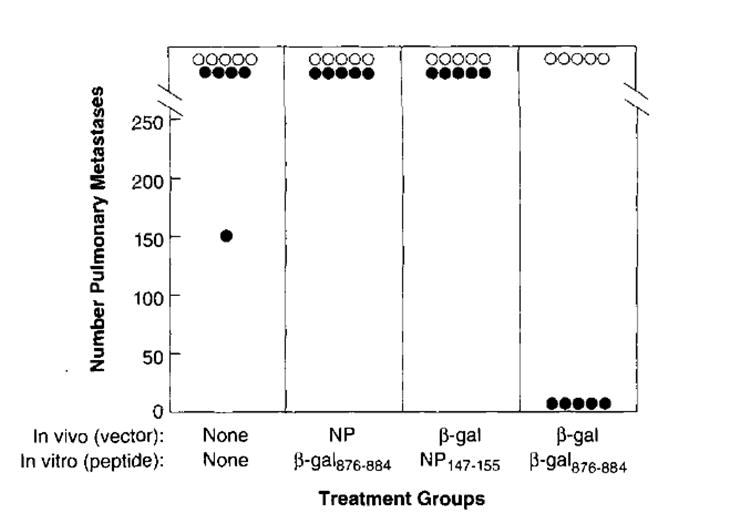
Adoptive immunotherapy of tumor-bearing mice with immune splenocytes induced by gene gun vaccination. On day 0, CT26.WT (β-gal−, ○) and CT26.CL25 β-gal+, ●) tumor cell lines were each injected i.v. into BALB/c mice to create lung metastases. On day 3, tumor-bearing mice were treated with effector splenocytes from donor mice. The donor cells were generated by prior gene gun immunization with 1 μg of either pCMV/β-gal or pCMV/NP followed 14 days later by in vitro incubation for 6 days with 1 μg/ml of either β-gal876–884 or NP147–155 peptide. On day 17, pulmonary metastases were enumerated in a coded, blind fashion. This experiment has been repeated one time with similar findings.
Gene gun immunization of DNA alone induces little to no active specific immunotherapy of established pulmonary metastases
Mice bearing 2-day established pulmonary metastases were immunized with Pcmv/β-gal to evaluate the ability of gene gun immunization of DNA to generate active specific therapeutic responses. Initial experiments were carried out in mice given 5 × 105 tumor cells as reported previously (44). Seven independent attempts to use gene gun immunization alone to treat established pulmonary metastases have been made with only one experiment demonstrating a reduction in tumor growth. When the number of tumor cells given was reduced to 105, significant reduction in the number of metastases or mouse survival was observed in groups receiving β-gal-specific gene gun immunization in two of three experiments performed (data not shown).
Cytokine administration following DNA vaccine leads to treatment of established pulmonary metastases
Several cytokines, known to have different immune-regulating effects, have been reported to have antitumor activity as single agent therapy (46-48, 51) as well as adjuvant to standard therapy (46, 52-54). For example, rhIL-2, rmIL-6, and rhIL-7 have been reported to activate or proliferate Ag-specific CTL populations (47, 55). GM-CSF promotes the differentiation of hematopoietic precursors to dendritic cells that function to present Ag to prime naive lymphocytes (56). rmIL-12, rmIL-4, and rmIL-10 have been shown to direct Th populations to different Th1 or Th2 phenotypes, resulting in shifts to either humoral or cell-mediated responses, respectively (57-60). A screening assay was performed to determine whether various cytokines could act as adjuvants to DNA vaccination to induce active specific immunotherapy. In these experiments, tumor-bearing mice immunized on day 2 with pCMV/βgal or unimmunized were treated 18 to 24 h later with either rhIL-2, rmIL-4, rmIL-6, rhIL-7, rmIL-10, rmIL-12, or rmGM-CSF administered exogenously. From this screening assay, only rhIL-2, rmIL-6, rhIL-7, and rmIL-12 were found to specifically induce Ag-specific active immunotherapy (the entire screen is not shown).
Figure 5 illustrates the average number of pulmonary metastases on day 12 following tumor challenge for those mice that received the DNA vaccine followed by either rhIL-2, rmIL-6, or rhIL-7 administration. Figure 5 represents the pooled averages of two separate experiments done with the same protocol. Mice that were injected with pCMV/β-gal alone demonstrated no significant reduction in the number of metastases (215) compared with control non-immunized mice (>250). The groups of mice that received pCMV/β-gal administration plus individual cytokines rhIL-2, rmIL-6, or rhIL-7 all demonstrated a significant reduction in number of metastases compared with either cytokine alone (all >250, p2 = 0.001, p2 = 0.003, and p2 = 0.0002, respectively), or β-gal alone (>250, p2 = 0.003, p2 = 0.0003, and p2 = 0.001, respectively).
FIGURE 5.
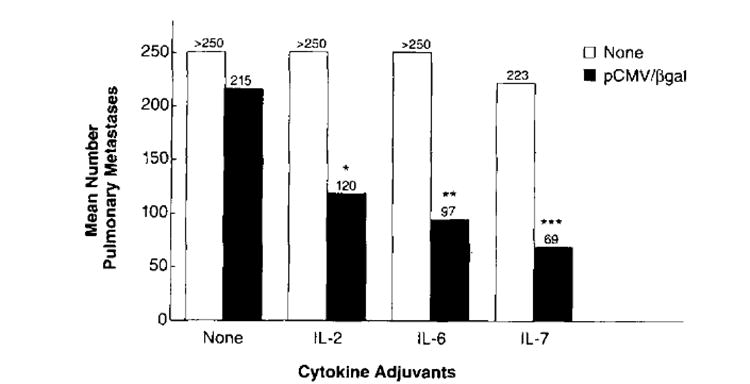
Active immunotherapy of established pulmonary metastases with the pCMV/β-gal vaccine plus systemic administration of rhIL-2, rmIL-6, or rhIL-7. BALB/c mice were injected i.v. with 5 × 105 CT26.CL25 (βgal+) tumor cells. On day 2 following tumor challenge, treated mice were immunized with 10 μg of pCMV/β-gal. Each mouse received 10 shots of 0.5 mg of gold, each shot delivering 1 μg of DNA. On day 3, mice (5 to 10 mice per group) began regimens of i.p. cytokine injections as described in Materials and Methods. On day 12, lungs were harvested and the pulmonary metastases were enumerated in a coded, blind fashion. Nonparametric statistical analysis was done using the Kruskal-Wallis test. The group with asterisks above were found to be statistically significant compared with either cytokine alone (*, p2 = 0.001; **, p2 = 0.003; ***, p2 = 0.0002) or to β-gal DNA alone (*, p2 = 0.003; **, p2 = 0.0003; ***, p2 = 0.001). The graph represents a summary of two experiments.
In an extensive experiment evaluating IL-12, treatment with pCMV/β-gal alone showed a slight but significant reduction in the number of pulmonary metastases compared with the group that received pCMV/NP (Fig. 6, 151 compared with 239, p2 = 0.016). This result was only observed one time out of seven experiments that were performed. rmIL-12 alone demonstrated a modest reduction in the tumor burden with an average of 177 ± 45 metastatic nodules compared with >250 in the untreated group. A dramatic reduction was observed with the combination of rmIL-12 and pCMV/β-gal (an average of 8 ± 2.5 metastases) (Fig. 6). This was significant in comparison to groups that received pCMV/β-gal alone (p2 = 0.012), rmIL-12 alone (p2 = 0.002), and pCMV/NP plus rmIL-12 (p2 = 0.008). pCMV/β-gal resulted in a significant reduction in tumor burden even with lower doses of rmIL-12. Doses of 0.3 and 0.1 μg of rmIL-12 were also able to diminish the tumor burden to 17 ± 3.8 and 32 ± 9.2 metastases, respectively. These data were significant compared with controls, pCMV/β-gal alone (p2 = 0.036 and p2 = 0.036, respectively) and rmIL-12 alone (p2 = 0.002 and p2 = 0.004, respectively). Therefore, when rmIL-12 is administered as an adjuvant with pCMV/β-gal DNA, lower doses of rmIL-12 can be given and therapeutic effects are still observed. This kind of active treatment experiment using IL-12 as an adjuvant to DNA immunization has been repeated three times with similar findings. Together, these studies show that cytokines rhIL-2, rmIL-6, rhIL-7, and rmIL- 12 given individually but in combination with pCMV/β-gal can lead to Ag-specific reduction in tumor burden.
FIGURE 6.
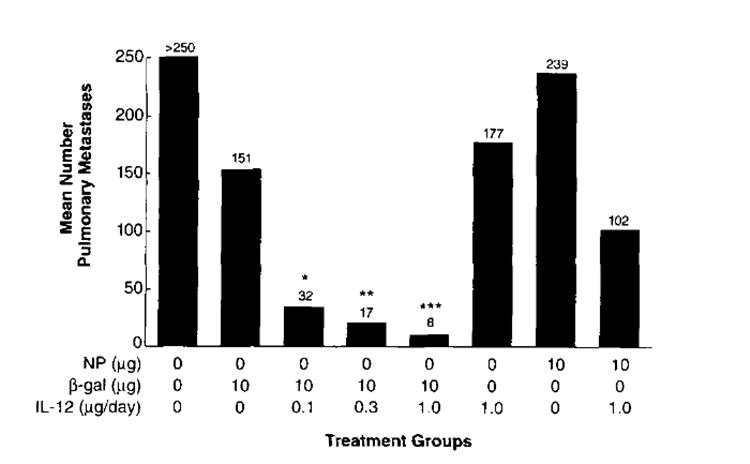
Active immunotherapy of established pulmonary metastases with the pCMV/β-gal vaccine plus systemic administration of rmIL-12. BALB/c mice were injected i.v. with 5 × 105 CT26.CL25 (βgal+) or CT26 (βgal−) tumor cells. On day 2 following tumor challenge, treated mice were immunized with 10 μg of pCMV/β-gal or 10 μg of pCMV/NP. Each mouse received 10 shots of 0.5 mg of gold, each shot delivering 1 μg of DNA. On day 3, mice began regimens of i.p. rmIL-12 injections as described in Materials and Methods. On day 12, lungs were harvested and the pulmonary metastases were enumerated in a coded, blind fashion. Nonparametric statistical analysis was done using the Kruskal-Wallis test. The groups with asterisks were found to be statistically significant compared with either cytokine alone (*, p2= 0.004; **, p2 = 0.002; ***, p2 = 0.002) or β-gal DNA (*, p2 = 0.036; **, p2 = 0.036; ***, p2 = 0.012) alone. The adjuvant effects of IL-12 have been observed three times, whereas the effect of β-gal has been observed only one time.
Discussion
To our knowledge, this study represents the first report of the use of gene gun immunization for the prevention and treatment of an experimental cancer. Plasmid DNA delivered by particle-mediated delivery induced potent humoral and CTL lytic immune responses that presumably aided in protective immunity from tumor challenge. Despite these potent responses, active immunity of established tumor with the DNA vaccination alone failed to have an impact on tumor burden. Therapeutic responses in tumor-bearing animals could be improved; however, only when particular cytokines (rhIL-2, rmIL-6, rhIL-7, and rmIL-12) were given following DNA administration.
DNA immunization resulted in potent Ag-specific Ab and CTL responses (Figs. 1 and 2). These findings were consistent with other reports of the use of DNA-based immunizations using either particle-mediated delivery to the epidermis or direct injection intramuscularly (17, 18, 20, 23-27). We opted for gene gun delivery of DNA based on studies that compared different routes of immunization. Fynan et al. demonstrated that the particle bombardment method required 250 to 2500 times less DNA than saline intramuscular inoculations to induce protective immunity from viral challenge (22). Similarly, Pertmer et al. found that direct inoculation of DNA intradermally or intramuscularly required 5000-fold more DNA to achieve comparable results (25). In our studies, as little as 0.1 to 0.01 μg of DNA was observed to induce potent and consistent CTL and Ab responses. In studies not shown, at least 50 μg administered intramuscularly would induce Ab and CTL responses, but inconsistently. However, we did not utilize muscle regeneration reagents such as bupivicaine (31), which have been reported to facilitate the uptake and transcription of the introduced DNA and therefore improve the immune responses observed.
Several other groups have described various direct injection DNA-based immunization protocols resulting in the prevention of not only tumor but also viral and nonviral diseases (22, 31-37). Recently, Conry et al. have developed a plasmid encoding full length cDNA for human CEA under the transcriptional control of the CMV early promoter/enhancer, which functioned to elicit CEA-specific humoral and cellular proliferation responses as well as protection against a syngeneic, CEA-expressing colon carcinoma cell line (35, 36). Wang et al. have also reported that the use of a plasmid encoding the envelope region of HIV (gp160) can induce long-lasting protection in mice from tumors expressing that gene (37).
In our study, DNA-based immunization was used to prime T lymphocytes reactive with the TAA before ex vivo expansion and adoptive immunotherapy, a strategy that was ineffective in the absence of in vivo priming. This represents a novel use of the gene gun approach and the strategy of using DNA as an immunogen for the treatment of established tumor. The poor performance of the DNA-based approach to treat established tumor in an active immunotherapy model suggested that the immune responses demonstrated in Figures 1and 2 were either not rapid enough in their kinetics or were below a threshold required to treat tumor. Unlike poxvirus-based vectors, DNA vaccines may not produce the quality of the antigenic protein required to elicit therapeutic antitumor responses in an active treatment regimen. The level of Ag production or persistence from the vaccine as well as the amounts of tumor Ag expressed by the tumor may be important for treatment. Increased amounts of recombinant adenovirus expressing a TAA were critical for the observation of any treatment effects (12a) Improved vectors that augment protein expression by the addition of enhancer elements are currently being designed (61).
Active treatment could be enhanced when cytokines were utilized as adjuvants to DNA immunization. When a panel of cytokines was tested, only rhIL-2, rmIL-6, rhIL-7, and rmIL-12 were able to enhance the therapeutic responses. rhIL-2, rhIL-7, and rmIL- 12 have all been reported to stimulate or activate T cell populations as well as NK cells (45, 46, 57). rmIL-12 also functions to regulate immune responses by directing a Th2 to a Thl phenotype (58, 60). IL-6, known as B cell-stimulatory factor-2, is a pleiotrophic cytokine that can enhance CTL function, NK activity, LAK, and TIL activity (47, 62). All of these cytokines have been reported to have antitumor effects when administered as single agent therapy (46-48, 63, 64). However, the doses used in this study were previously shown to have little to no effect on the growth of CT26.CL25.4 On the other hand, cytokines such as GM-CSF, IFN-γ, and IL-4, when given in the adjuvant setting, failed to mediate tumor regression. IL-4 may not have had an effect because it steers the T helper cell population toward a Th2 phenotype responsible for the enhancement of humoral responses but not of cell mediated immunity (65). IFN-γ up-regulates the production of key molecules involved in Ag processing and presentation of intracellular Ags (66) but may also have an antiproliferative effect on T cells GM-CSF was reported in earlier studies to have little to no adjuvant effect when encoded in the same recombinant virus as TAA (44). We have also observed little to no adjuvant effect when GM-CSF was administered exogenously with a rVV encoding β-gal in three of four experiments.4 The findings in this study, that GM-CSF did not act an adjuvant, suggested that the APC are not limiting when the Ag supplied by DNA immunization.
The known functions of the cytokines that acted as adjuvants compared with those that failed to enhance therapeutic responses suggest that proliferation and activation of primed lymphocytes are important mechanisms for active treatment to occur. The use of cytokines to enhance tumor immunity has been reported previously in a number of different vaccine settings. Sun et al. have shown that gene gun injection of cytokines rmIL-6, rhIL2, and rmTNF-α directly into the tumor mediate tumor reduction (67). Bronte et al. have shown that either exogenous administration of rhIL-2 with rVV or rhIL-2 plus Ag β-gal encoded within the vaccinia viral genome can also enhance treatment responses (44) Rao et al. have observed that other cytokines, rmIL-12 and rmIL-10, administered exogenously along with rVV-encoding β-gal can also augment therapeutic responses.4 In addition, rhIL-2, rmIL-6, rhIL-7, and rmIL-12 have each been reported previously to have adjuvant effects in different Ag settings (46, 52-54, 68).
With the particle delivery technique, several genes encoding different molecules can be coated onto each gold bead; thus each bombarded cell could potentially elaborate more than one protein. Recently, Xiang and Ertl demonstrated immune enhancement by co-injecting plasmids encoding Ag along with plasmids encoding murine cytokine genes (69). Specifically, intramuscular injection of DNA encoding GM-CSF with DNA encoding rabies glycoprotein could augment the rabies-specific Ab, as well as enhanced protection from viral challenge. In contrast, the addition of a vector containing IFN-γ did not induce increased Ab production, T cell proliferation, or protective immunity from viral challenge (69). Future studies will be directed at the development of beads that are coated with DNA encoding immune-regulating molecules as well as Ag to determine whether we can improve the antitumor immune effects.
It should be emphasized that β-gal is a molecule foreign to a mouse, and the responses elicited by the pCMV/β-gal constructs may not be the same as the responses induced by a self-Ag. However, it is intriguing that transfection of tumors with several nonself-genes, including human CEA, NP from VSV virus, and E. coli-derived β-gal, do not result in the rejection of the experimental tumor (11, 12, 70). In fact, CT26.WT and CT26.CL25 grow at equivalent rates and no secondary anti-β-gal-lytic responses have been detected in CT26.CL25-bearing mice (12). However, these nonself-Ags do bear similarities to some potential tumor Ags such as viral Ags expressed in virally induced tumors, mutated tumor suppressor genes(71), fusion proteins resulting from translocations (72), frame-shifts,5 and loss of stop codons. Since many tumor Ags reported thus far seem to be nonmutated differentiation Ags (4-9), further studies will be aimed at evaluating effects of tolerance by either using mice transgenic for the β-gal gene or by immunization with genes encoding self-Ags within murine tumors such as the P815 mastocytoma-encoded P1A gene, and the murine homologues of the human melanoma Ags (73).
One safety issue of vaccination with nucleic acids includes the possibility of inducing an anti-DNA Ab response, which potentially could result in an autoimmune disease such as systemic lupus erythematosus. Although it is possible to generate Abs using denatured ssDNA as an immunogen, it has been shown to be difficult to induce Abs against dsDNA. In one study, primates were immunized by intramuscular injection several times, and anti-DNA Abs were not found (74). A second issue of DNA immunization is the potential integration of injected DNA into the host cell genome that may result in a transformation event. Wolff et al. addressed this point in one study in which more than 1800 recloned plasmids were analyzed for junctions between chromosomal and plasmid DNA extracted from muscle derived from mice previously immunized with DNA by direct injection (28). No evidence of DNA integration was observed. Of course, these issues will have to be addressed for particle-bombarded administration of DNA as well.
DNA-based vaccines offer several advantages over the use of recombinant viruses for immunization. Purified DNA is relatively safe compared with replication-competent viruses, which may result in disseminated viremia especially in immunocompromised individuals (13, 14, 75). Plasmid DNA can also be easily and rapidly purified in comparison to the production of live viruses, which involves time-consuming homologous recombination and plaque purification steps. The use of DNA vectors would also eliminate the problems of anamnestic responses that can eliminate recombinant viruses more rapidly, thus reducing immune responses against heterologous proteins expressed by these viral carriers (14-16). Genetic vaccines followed by cytokine treatment represent a safe alternative to recombinant viral vaccines for the evaluation of the immunotherapy of cancer.
Acknowledgments
The authors thank Jim Yang, Vincenzo Bronte, and Patrick Hwu for important discussion and ideas. We also thank Joel Haynes, Marty Ford, Deborah Fuller, and Joseph Burkholder at Agracetus, Inc. for aid with the operation of the gene gun, as well as for valuable reagents, and Paul Spiess, Dave Jones, and Eliza Shulman for technical assistance. We also greatly appreciate the advice and rmIL-12 given to us by Stan Wolf at Genetics Institute Inc.
Abbreviations used in this paper
- TAA
tumor-associated Ag
- β-gal
β-galactosidase
- NP
nucleoprotein
- CEA
carcinoembryonic Ag
- rm
recombinant murine
- rh
recombinant human
- hGH
human growth hormone
- CM
complete medium
- BID
twice dally
- QD
once daily
- GM-CSF
granulocyte macrophage-CSF
- rVV
recombinant vaccine virus
Footnotes
Rao, J . B., V. Bronte, K. R. Irvine, M. W. Carroll, S. A. Rosenberg, and N. P. Restifo. Submitted for publication.
R. Wang et al. Utilization of an alternative open reading frame of a normal gene in generating a novel human cancer antigen. Submitted for publication.
References
- 1.Greenberg PD, Cheever MA, Fefer A. H-2 restriction of adoptive immunotherapy of advanced tumors. J Immunol. 1981;126:2100. [PubMed] [Google Scholar]
- 2.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwnrrzentruber D, Wei JP, White DE. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: preliminary report. N Engl J Med. 1988;319:1676. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 4.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocyres on HLA-A2 melanomas. J Exp Med. 1993;178:489. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264(716) doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohbins PF, El-Gamil M, Li YF, Topalian SL, Rivoltini L, Sakaguchi K, Appella E, Kawakami Y, Rosenberg SA. Cloning of a new gene encoding an antigen recognized by melanoma-specific HLA-A24-restricted tumor-intiltrating lymphocytes. J Immunol. 1995;154:5944. [PubMed] [Google Scholar]
- 9.Pardoll DM. A new look for the 1990’s. Science. 1995;369:357. [Google Scholar]
- 10.Estin CD, Stevenson US, Plowman GD, Hu S-L, Sridham P, Hellstrom I, Brown JP, Hellstrom KE. Recombinant vaccinia virus against the human melanoma antigen p97 for use in Immunotherapy. Proc Natl Acad Sci USA. 1988;85:1052. doi: 10.1073/pnas.85.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J lmmunol. 1995;154:4685. [PMC free article] [PubMed] [Google Scholar]
- 12a.Chen PW, Wang M, Bronte V, Zhai Y, Rosenherg SA, Restifo NP. Therapeutic anti-tumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J lmmunol. 1996 In press. [PMC free article] [PubMed] [Google Scholar]
- 13.McElrath MJ, Corey L, Berger D, Hoffman MC, Klucking S, Dragavon J, Peterson E, Greenberg PD. Immune responses elicited by recombinant vaccinia-human immunodeficiency virus (HIV)envelope and HIV envelope protein: analysis of the durability of responses and effect of repeated boosting. J Infect Dis. 1994;169:4l. doi: 10.1093/infdis/169.1.41. [DOI] [PubMed] [Google Scholar]
- 14.Graham BS, Matthews TJ, Belshe RB, Clements ML, Dolin R, Wright PF, Gorse GJ, Schwartz DH, Keefer MC, Bolognesi DP. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgpl60 in vaccinia-naive adults: the NIAID AIDS Vaccine Clinical Trials Network. J Infect Dis. 1993;167:533. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 15.Battegay M, Moskophidis D, Waldner H, Brundler MA, Fung-Leung WP, Mak TW, Hengartner H, Zinkernagel RM. Impairment and delay of neutralizing antiviral antibody responses by virus-specitic cytotoxic T cells. J Immunol. 1993;151:5408. [PubMed] [Google Scholar]
- 16.Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, Hoffman MC, Hu SL, Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoproteins. Lancet. 1991;337:567. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 17.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct transfer into mouse muscle in vivo. Science. 1990;247:1465. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 18.Davis HL, Whalen RG, Demeneix BA. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther. 1993;4:151. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 20.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang N-S, Burkholder J, Roberts B, Martinelli B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci USA. 1990;87:9568. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RS, Johnston SA, Riedy M, DeVit MJ, McElligott SG, Sanford JC. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci USA. 1991;88:2726. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fynan E, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parental, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1995;90:11478. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenbraun MD, Fuller DH, Haynes JR. Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization. DNA Cell Biol. 1993;12:791. doi: 10.1089/dna.1993.12.791. [DOI] [PubMed] [Google Scholar]
- 24.Fuller DH, Haynes JR. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res Hum Retroviruses. 1994;10:1433. doi: 10.1089/aid.1994.10.1433. [DOI] [PubMed] [Google Scholar]
- 25.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995 doi: 10.1016/0264-410x(95)00069-d. In press. [DOI] [PubMed] [Google Scholar]
- 26.Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A, Wolff JA, Davies KE. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991;352:815. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- 27.Vitadello M, Schiaffino MV, Picard A, Scarpa M, Schiaffino S. Gene transfer in regenerating muscle. Hum Gene Ther. 1994;5:11. doi: 10.1089/hum.1994.5.1-11. [DOI] [PubMed] [Google Scholar]
- 28.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 29.Tang D, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 30.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowsi SH, Deck RR, DeWitt CM, Friedman A, Hawe LA, Leander KR, Martinez D, Perry HC, Shiver JW, Montgomery DL, Lui MA. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Ugen EU, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, Sato A, Boyer J, Williams WV, Weiner DB. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sedegah M, Hedstrom R, Hobart P, Hogman SL. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1995;91:9866. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedroza Martins L, Lau LL, Asano MS, Ahmed R. DNA vaccination against persistent viral infection. J Virol. 1995;69:2574. doi: 10.1128/jvi.69.4.2574-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarozinski CC, Fynan EF, Selin LK, Robinson H, Welsh RM. Protective CTL-dependent immunity and enhanced immunopathology in mice immunized by particle bombardment with DNA encoding an internal virion protein. J Immunol. 1995;154:4010. [PubMed] [Google Scholar]
- 35.Conry RM, LoBuglio AF, Kantor J, Schlom J, Loechel F, Moore SE, Sumerel LA, Barlow DL, Abrams S, Curiel DT. Immune response to a carcinoembryonic antigen polynucleotide vaccine. Cancer Res. 1994;54:1164. [PubMed] [Google Scholar]
- 36.Conry RM, LoBuglio AF, Loechel F, Moore SE, Sumerel LA, Barlow DL, Curiel DT. A carcinoembryonic antigen polynucleotide vaccine has in vivo antitumor activity. Gene Ther. 1995;2:59. [PubMed] [Google Scholar]
- 37.Wang B, Merva M, Dang K, Ugen K, Boyer J, Williams WV, Weiner DB. DNA inoculation induces protective in vivo immune responses against cellular challenge with HIV-1 antigen expressing cells. AIDS Res Hum Retroviruses. 1994;10:21. [PubMed] [Google Scholar]
- 38.Brattain MG, Strobel-Stevens J, Fine D, Webb M, Sarrif AM. Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 1980;40:2142. [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 40.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recomhinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971. [PubMed] [Google Scholar]
- 41.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mulé JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Restifo NP, Esquivel F, Asher AL, Stötter H, Barth RJ, Bennink JR, Mulé JJ, Yewdell JW, Rosenberg SA. Defective presentation of endogenous antigens by a murine sarcoma: implications for the failure of an anti-tumor immune response. J Immunol. 1991;147:1453. [PMC free article] [PubMed] [Google Scholar]
- 43.Mulé JJ, Yang JC, Lafrenière R, Shu S, Rosenberg SA. Identiticatlon of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by the systemic administration of high-dose recombinant interleukin-2. J Immunol. 1987;139:285. [PubMed] [Google Scholar]
- 44.Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, Restifo NP. IL-2 enhancesthe function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J lmmunol. 1995;154:5282. [PMC free article] [PubMed] [Google Scholar]
- 45.Jicha DL, Mulé JJ, Rosenberg SA. Interleukin 7 generates antitumor cytotoxic T lymphocytes against murine sarcomas with efficacy in cellular adoptive immunotherapy. J Exp Med. 1991;174:1511. doi: 10.1084/jem.174.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komschlies KL, Gregorio TA, Gruys ME, Back TC, Faltynek CR, Wiltrout RH. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J lmmunol. 1994;153:5776. [PubMed] [Google Scholar]
- 47.Mulé JJ, Custer MD, Travis WD, Rosenberg SA. Cellular mechanisms of the antitumor activity of recombinant IL-6 in mice. J lmmunol. 1992;148:2622. [PubMed] [Google Scholar]
- 48.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill AD, Redmond HP, Austin OM, Grace PA, Bouchier-Hayes D. Granulocyte-macrophage colony-stimulating factor inhibits tumour growth. Br J Surg. 1993;80:1543. doi: 10.1002/bjs.1800801216. [DOI] [PubMed] [Google Scholar]
- 50.Topp MS, Koenigsmann M, Mire-Sluis A, Oberberg D, Eitelbach F, von Marschall Z, Notter M, Reufi B, Stein H, Thiel E. Recombinant human interleukin-4 inhibits growth of some human lung tumor cell lines in vitro and in vivo. Blood. 1993;82:2837. [PubMed] [Google Scholar]
- 51.Tahara H, Lotze M. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995;2:96. [PubMed] [Google Scholar]
- 52.Takatsuki F, Okano A, Suzuki C, Chieda R, Takahara Y, Hirano T, Tadamitsu K, Hamuro J, Akiyama Y. Human IL 6/B cell stimulatory factor-2 augments murine antigen-specific antibody responses in vitro and in vivo. J Immunol. 1988;141:3072. [PubMed] [Google Scholar]
- 53.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 54.Noguchi Y, Richards EC, Chen Y-T, Old LJ. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci USA. 1995;92:2219. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jicha DL, Mulé JJ, Rosenberg SA. Interleukin 7 generates antitumor cytotoxic T lymphocytes against murine sarcomas with efficacy in cellular adoptive immunotherapy. J Exp Med. 1991;174(151l) doi: 10.1084/jem.174.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegal JP. Effects of IL-12 on the generation of cytotoxic activity on human CD8+ T lymphocytes. J Immunol. 1993;151:2444. [PubMed] [Google Scholar]
- 58.Hsieh CS, Macatonia SE, Triipp CS, Wolf SF, O’Garra A, Murphy KM. Development of Thl CD4+ T cells through IL-12 produced Listeria-induced macrophages. Science. 1993;260:547. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 59.Bertagnolli MM, Lin B-Y, Young D, Herrmann SH. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992;149:3778. [PubMed] [Google Scholar]
- 60.Sypek JP, Chung CL, Mayor SH, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapman BS, Thayer RM, Vincent KA, Haigwood N. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;19:3979. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takai Y, Wong GG, Clark SC, Burkoff SJ, Herrmann SH. B-cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J lmmunol. 1988;140:508. [PubMed] [Google Scholar]
- 63.Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM, Schwarz S. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high dose recombinant IL-2. J Exp Med. 1985;161:1169. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radrizzani M, Accornero P, Amidei A, Aeillo A, Delia D, Kurrle R, Colombo MP. IL-12 inhibits apoptosis induced in a human Thl clone by gp120/CD4 crosslinking and CD3/TCR activation or by IL-2 deprivation. Cell lmmunol. 1995;161:1421. doi: 10.1006/cimm.1995.1003. [DOI] [PubMed] [Google Scholar]
- 65.Mosmann TR. Cytokine secretion patterns and cross-regulation of T cell subsets. Immunol Res. 1991;10:183. doi: 10.1007/BF02919690. [DOI] [PubMed] [Google Scholar]
- 66.Restifo NP, Spiess PJ, Karp SE, Mulé JJ, Rosenberg SA. A nonimmunogenic sarcoma transduced with the cDNA for interferon γ elicits CD8+ T cells against the wild-type tumor: correlation with antigen presentation capability. J Exp Med. 1992;175:1423. doi: 10.1084/jem.175.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun WH, Burkholder JK, Sun J, Culp J, Turner J, Lu XG, Pugh TD, Ershler WB, Yang N-S. In vivo cytokine gene transfer by gene gun reduces tumor growth in mice. Proc Natl Acad Sci USA. 1995;92:2889. doi: 10.1073/pnas.92.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang Z-E, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 69.Xiang Z, Ertl HCJ. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 70.Kundig TM, Bachmann MF, Lefrancois L, Puddington L, Hengartner H, Zinkernagel RM. Nonimmunogenic tumor cells may efficiently restimulate tumor antigen-specific cytotoxic T cells. J lmmunol. 1993;150:4450. [PubMed] [Google Scholar]
- 71.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 72.Gribben JG, Saporito L, Barber M, Blake KW, Edwards RM, Griffin JD, Freedman AS, Nadler LM. Bone marrows of non-Hodgkin’s lymphoma patients with a bcl-2 translocation can be purged of polymerase chain reaction-detectable lymphoma cells using monoclonal antibodies and immuno-magnetic bead depletion. Blood. 1992;80:1083. [PubMed] [Google Scholar]
- 73.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J Exp Med. 1991;173:1373. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiao SP, Williams P, Berg RK, Hodgeman BA, Liu L, Repetto G, Wolff JA. Direct gene transfer into nonhuman primate myofibers in vivo. Hum Gene Ther. 1992;3:21. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 75.Johnson RP, Hammond SA, Trocha A, Siliciano RF, Walker BD. Induction of a major histocompatibihty complex class I-restricted cytotoxic T-lymphocyte response to a highly conserved region of human immunodeficiency virus type 1 (HIV- I) gp120 in seronegative humans immunized with a candidate HIV-I vaccine. J Virol. 1994;68:3145. doi: 10.1128/jvi.68.5.3145-3153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


