Abstract
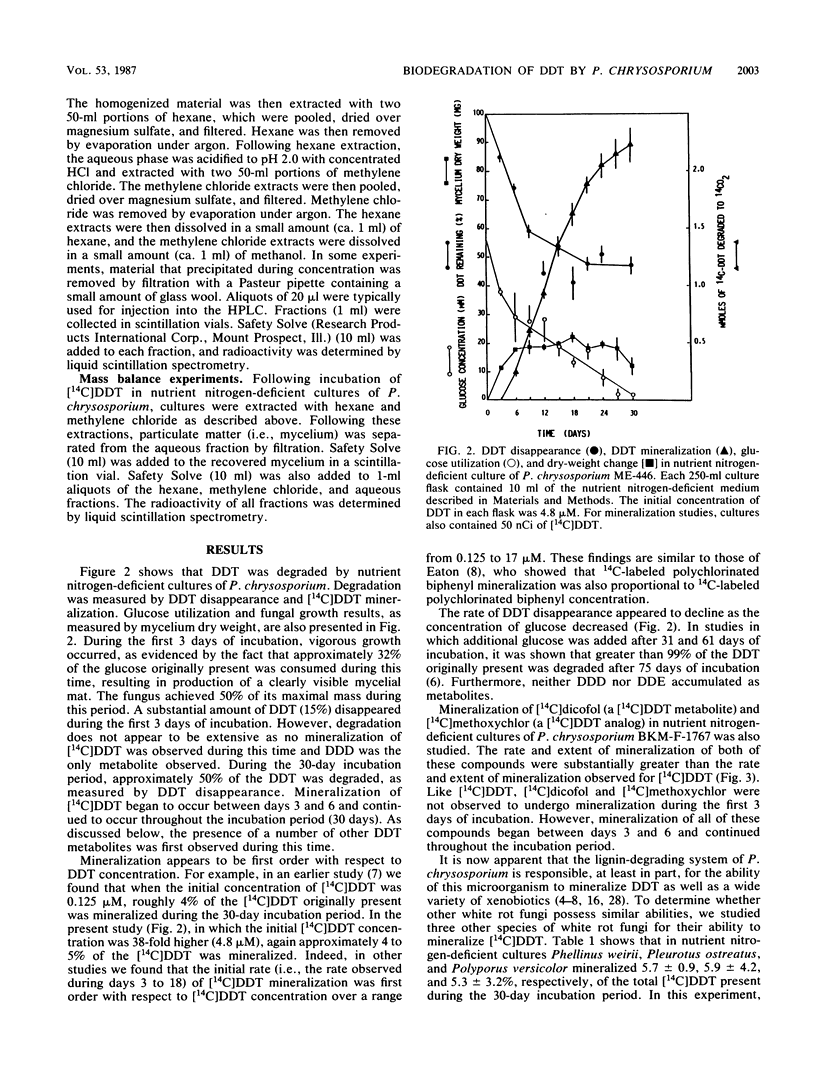
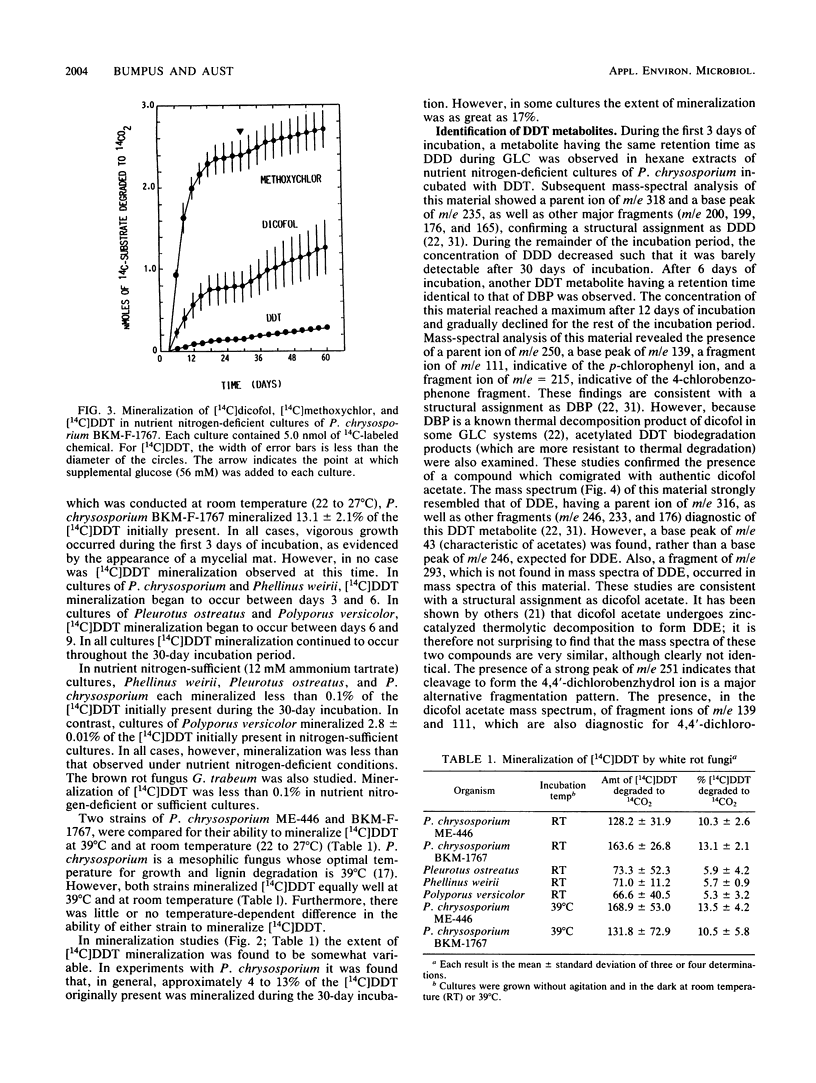
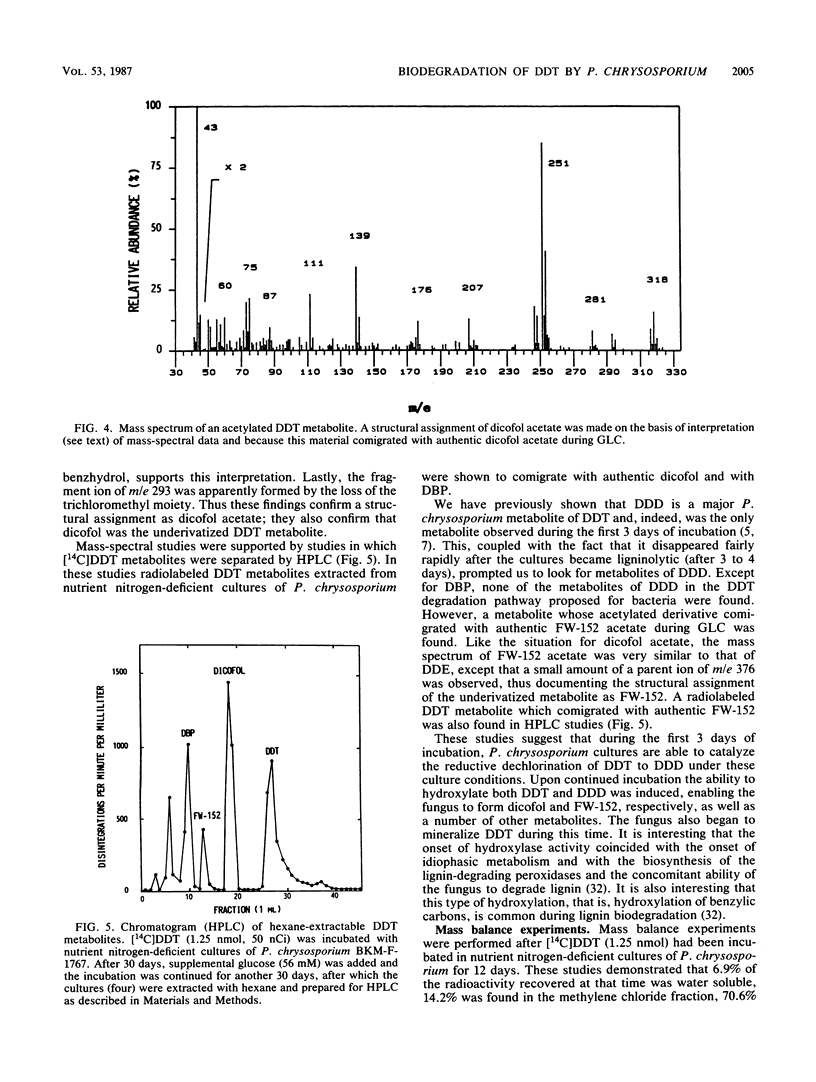
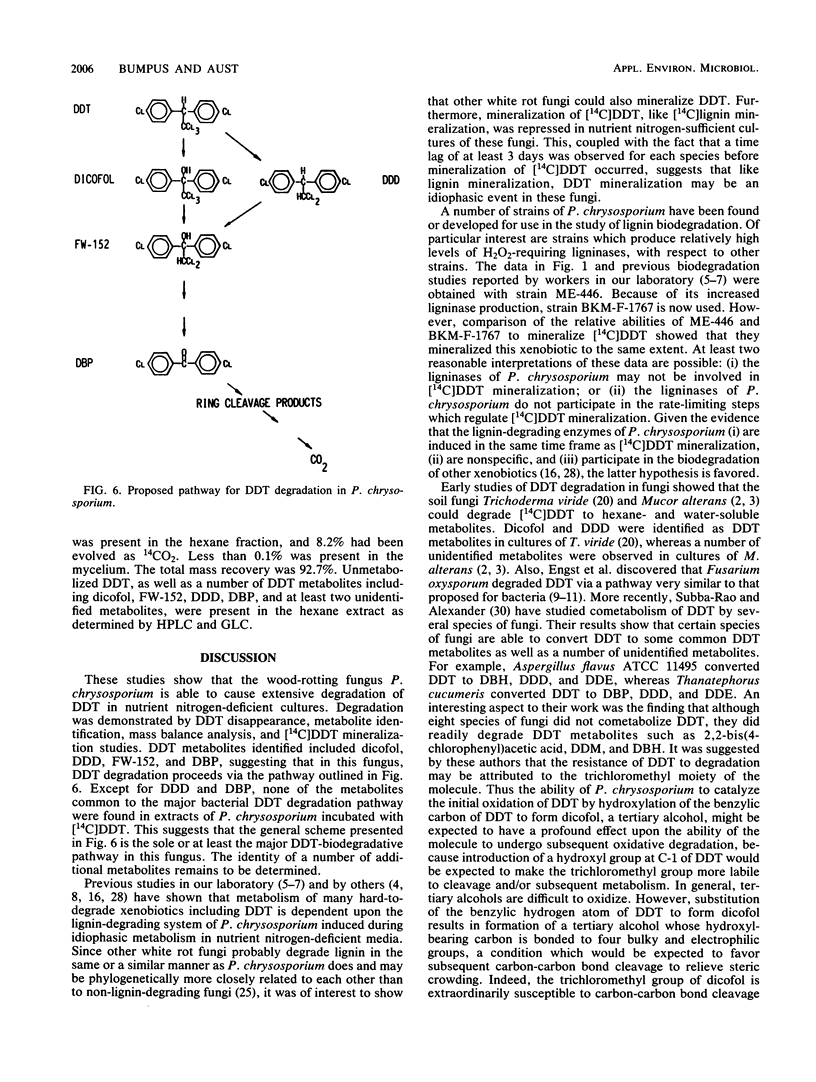
Extensive biodegradation of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) by the white rot fungus Phanerochaete chrysosporium was demonstrated by disappearance and mineralization of [14C]DDT in nutrient nitrogen-deficient cultures. Mass balance studies demonstrated the formation of polar and water-soluble metabolites during degradation. Hexane-extractable metabolites identified by gas chromatography-mass spectrometry included 1,1,-dichloro-2,2-bis(4-chlorophenyl)ethane (DDD), 2,2,2-trichloro-1,1-bis(4-chlorophenyl)ethanol (dicofol), 2,2-dichloro-1,1-bis(4-chlorophenyl)ethanol (FW-152), and 4,4'-dichlorobenzophenone (DBP). DDD was the first metabolite observed; it appeared after 3 days of incubation and disappeared from culture upon continued incubation. This, as well as the fact that [14C]dicofol was mineralized, demonstrates that intermediates formed during DDT degradation are also metabolized. These results demonstrate that the pathway for DDT degradation in P. chrysosporium is clearly different from the major pathway proposed for microbial or environmental degradation of DDT. Like P. chrysosporium ME-446 and BKM-F-1767, the white rot fungi Pleurotus ostreatus, Phellinus weirii, and Polyporus versicolor also mineralized DDT.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Lichtenstein E. P. Effect of nutritional factors on DDT-degradation by Mucor alternans. Can J Microbiol. 1971 Oct;17(10):1291–1298. doi: 10.1139/m71-208. [DOI] [PubMed] [Google Scholar]
- Anderson J. P., Lichtenstein E. P., Whittingham W. F. Effect of Mucor alternans on the persistence of DDT and Dieldrin in culture and in soil. J Econ Entomol. 1970 Oct;63(5):1595–1599. doi: 10.1093/jee/63.5.1595. [DOI] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. Aerobic cometabolism of DDT analogues by Hydrogenomonas sp. J Agric Food Chem. 1971 Jan-Feb;19(1):20–22. doi: 10.1021/jf60173a042. [DOI] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. Bacterial degradation of diphenylmethane, a DDT model substrate. Appl Microbiol. 1970 Oct;20(4):608–611. doi: 10.1128/am.20.4.608-611.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. DDT metabolites and analogs: ring fission by Hydrogenomonas. Science. 1970 Oct 2;170(3953):91–92. doi: 10.1126/science.170.3953.91. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Matsumura F., Boush G. M. Degradation of insecticides by a soil fungus, trichoderma viride. J Econ Entomol. 1968 Jun;61(3):610–612. doi: 10.1093/jee/61.3.610. [DOI] [PubMed] [Google Scholar]
- Pfaender F. K., Alexander M. Extensive microbial degradation of DDT in vitro and DDT metabolism by natural communities. J Agric Food Chem. 1972 Jul-Aug;20(4):842–846. doi: 10.1021/jf60182a045. [DOI] [PubMed] [Google Scholar]
- Subba-Rao R. V., Alexander M. Bacterial and fungal cometabolism of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) and its breakdown products. Appl Environ Microbiol. 1985 Mar;49(3):509–516. doi: 10.1128/aem.49.3.509-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Alexander M. Products Formed from Analogues of 1,1,1-Trichloro-2,2-Bis(p-Chlorophenyl) Ethane (DDT) Metabolites by Pseudomonas putida. Appl Environ Microbiol. 1977 Jan;33(1):101–108. doi: 10.1128/aem.33.1.101-108.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. R., Hites R. A. Dicofol solubility and hydrolysis in water. Bull Environ Contam Toxicol. 1979 Jun;22(3):305–311. doi: 10.1007/BF02026947. [DOI] [PubMed] [Google Scholar]
- Wedemeyer G. Biodegradation of Dichlorodiphenyltrichloroethane: Intermediates in Dichlorodiphenylacetic Acid Metabolism by Aerobacter aerogenes. Appl Microbiol. 1967 Nov;15(6):1494–1495. doi: 10.1128/am.15.6.1494-1495.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer G. Dechlorination of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane by Aerobacter aerogenes. I. Metabolic products. Appl Microbiol. 1967 May;15(3):569–574. doi: 10.1128/am.15.3.569-574.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


